Btk Inhibitors And Multiple Sclerosis
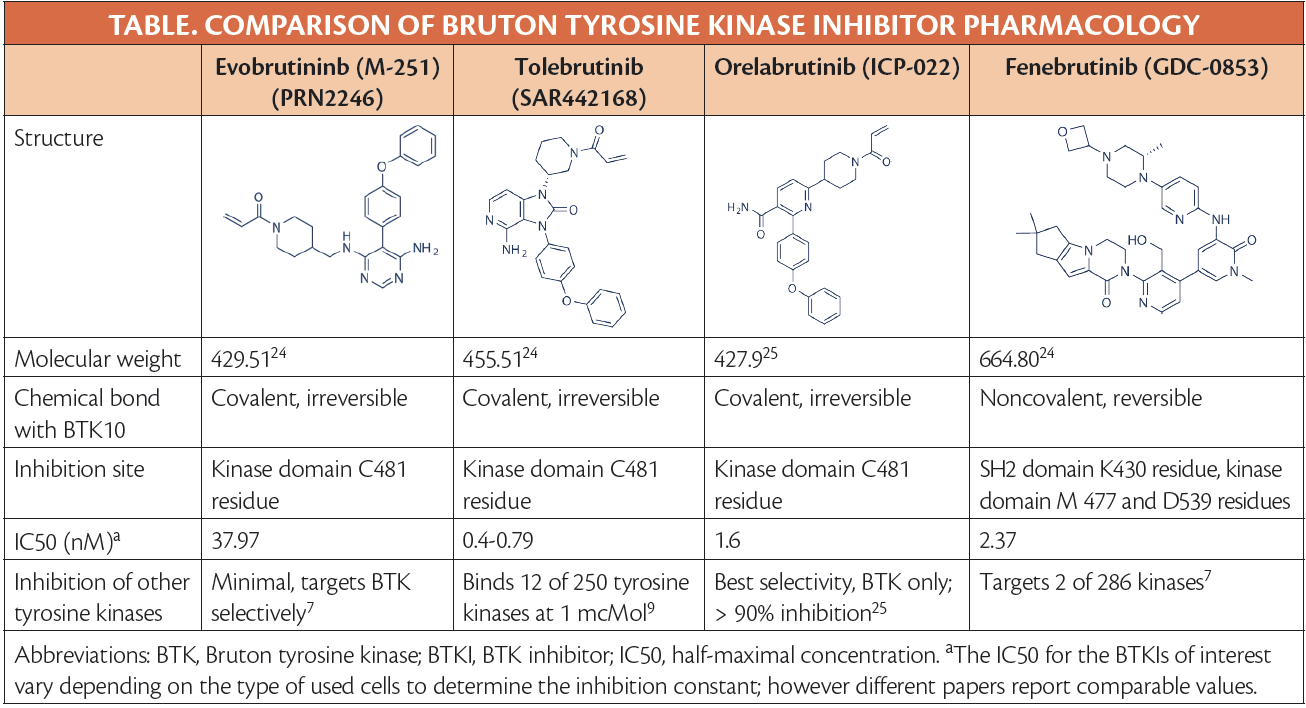
Bruton Tyrosine Kinase Inhibition In Multiple Sclerosis Practical A year of the experimental treatment fenebrutinib suppressed nearly all disease activity in people with relapsing MS in a clinical trial Roche’s fenebrutinib this week scored a mid-stage win in relapsing multiple sclerosis, while Sanofi’s tolebrutinib met the primary endpoint in a Phase III trial for progressive MS but flopped in two
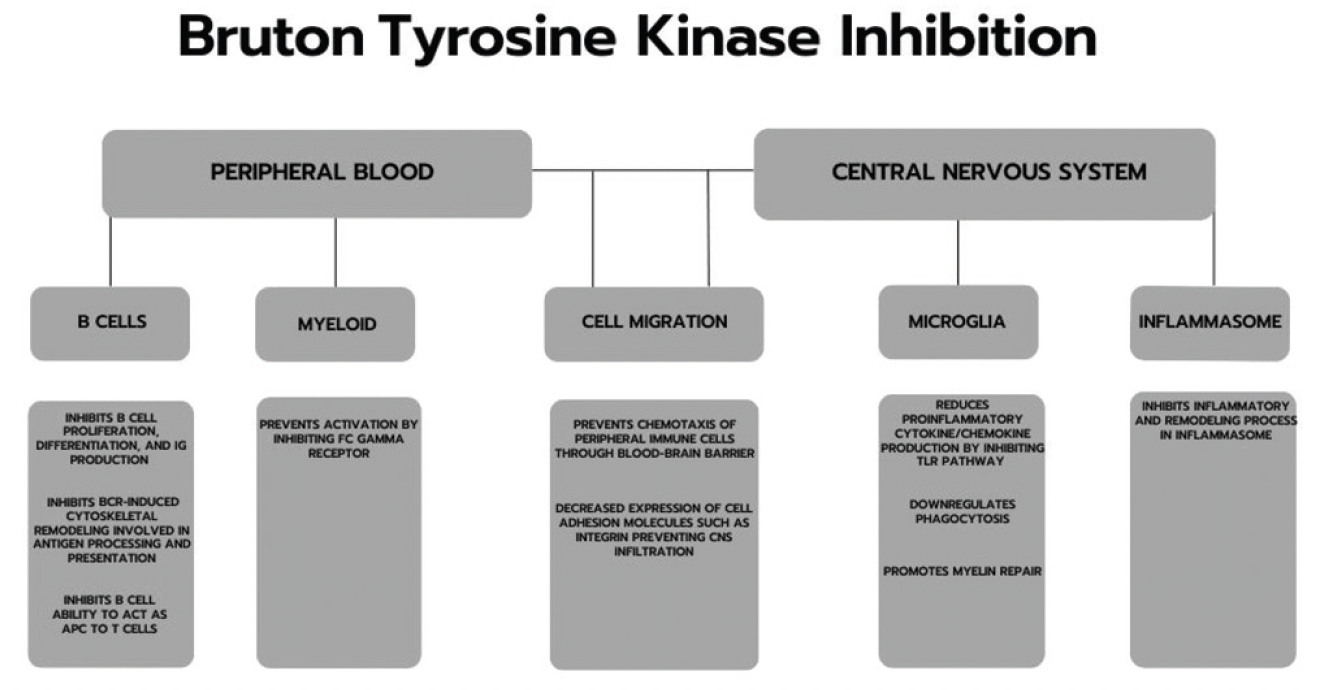
Updates In Bruton Tyrosine Kinase Inhibition For Multiple Sclerosis Dr Oh looks forward to new developments in disease-modifying therapy for relapsing-remitting multiple sclerosis A key focus will be the results from large phase 3 trials of BTK inhibitors such as Sanofi is still set on taking its multiple sclerosis (MS) med tolebrutinib to the FDA, executives have told Fierce Biotech, despite the BTK inhibitor falling short in two of three phase 3 trials that Tolebrutinib, which Sanofi acquired in a $37 billion deal, failed two studies in people with relapsing disease, but succeeded in a type of multiple sclerosis that has no approved therapies Tolebrutinib was one of three oral BTK inhibitors that Sanofi acquired as part of its $37 billion buyout of Principia Biopharma in 2020, and was abandoned as a treatment for myasthenia gravis -

Evobrutinib Is The First And Only Btk Inhibitor For Multiple Sclerosis Tolebrutinib, which Sanofi acquired in a $37 billion deal, failed two studies in people with relapsing disease, but succeeded in a type of multiple sclerosis that has no approved therapies Tolebrutinib was one of three oral BTK inhibitors that Sanofi acquired as part of its $37 billion buyout of Principia Biopharma in 2020, and was abandoned as a treatment for myasthenia gravis - Roche has announced positive new 48-week data for investigational fenebrutinib in patients with relapsing forms of multiple sclerosis (MS) The phase 2 FENopta open-label extension (OLE) study (Reuters) - Sanofi's most advanced multiple sclerosis (MS) drug candidate has missed the main goal of two late-stage trials to treat relapsing forms of the disease, dimming the prospects for a Results demonstrate that patients with relapsing multiple sclerosis (RMS) treated with fenebrutinib for up to one year maintained very low levels of disease activity and no disability progression

Updates In Bruton Tyrosine Kinase Inhibition For Multiple Sclerosis Roche has announced positive new 48-week data for investigational fenebrutinib in patients with relapsing forms of multiple sclerosis (MS) The phase 2 FENopta open-label extension (OLE) study (Reuters) - Sanofi's most advanced multiple sclerosis (MS) drug candidate has missed the main goal of two late-stage trials to treat relapsing forms of the disease, dimming the prospects for a Results demonstrate that patients with relapsing multiple sclerosis (RMS) treated with fenebrutinib for up to one year maintained very low levels of disease activity and no disability progression

Comments are closed.