Calculating Frequency Wavelength And Energy Practice Problems Docx

Calculating Frequency Wavelength And Energy Practice Problems Docx To print or download this file, click the link below: wavelength frequency practice problems w key.docx — application vnd.openxmlformats officedocument. In these practice problems, we will go over examples of determining the wavelength, frequency, and energy of light, calculating the number of photons in a laser pulse based on the energy, understanding the correlation between the energy and wavelength and frequency of electromagnetic radiation, examples of calculating the energy of transitions based on the bohr’s model of the hydrogen atom.

Calculating Frequency Wavelength And Energy Worksheets Study with quizlet and memorize flashcards containing terms like calculate the wavelength given the frequency of radiation is 6.10 x 10^14 hz., what is the energy of violet light with a frequency= 7.50x 10^14 hz?, what is the energy of cell phone radiation with a 1000 m wavelength? and more. E = energy (j) = wavelength (m) ! = frequency (hz or s 1) h = planck’s constant, 6.626x10 34 j∙s c = the speed of light in a vacuum, 3.00 × 108 m∙s 1 during the course of this unit, you should become very comfortable with the process of solving problems like the following. you may also want to review scientific prefixes. Calculate the wavelength and energy of light that has a frequency of 1.5 x 1015 hz. ans: = 2.0 x 10 7 m e = 9.95 x 10 19 j. a photon of light has a wavelength of 0.050 cm. calculate its energy. ans: e = 3.98 x 10 22 j. calculate the number of photons having a wavelength of 10.0 m required to produce 1.0 kj of energy. ans: 5.0 x 1022 photons. 4. a radio station broadcasts am fm with a frequency of 9.47 x 105 hz. calculate the wavelength and energy of the broadcast. 5. calculate the energy of a ultraviolet photon with a wavelength of 1.18 x 10 8 m. 6. calculate the frequency of a photon, which has an energy of 2.93 x 10 25 j. 7. calculate the wavelength of a photon that has an energy.

Calculating Frequency Wavelength And Energy Worksheet Answer Key Fill Calculate the wavelength and energy of light that has a frequency of 1.5 x 1015 hz. ans: = 2.0 x 10 7 m e = 9.95 x 10 19 j. a photon of light has a wavelength of 0.050 cm. calculate its energy. ans: e = 3.98 x 10 22 j. calculate the number of photons having a wavelength of 10.0 m required to produce 1.0 kj of energy. ans: 5.0 x 1022 photons. 4. a radio station broadcasts am fm with a frequency of 9.47 x 105 hz. calculate the wavelength and energy of the broadcast. 5. calculate the energy of a ultraviolet photon with a wavelength of 1.18 x 10 8 m. 6. calculate the frequency of a photon, which has an energy of 2.93 x 10 25 j. 7. calculate the wavelength of a photon that has an energy. 3.0928 x 10⁻3 m. what is the frequency of a wave that has a wavelength = 97 nm? 3.09 x 10¹⁵ hz. what is the frequency of a wave that has a wavelength of 0.07 cm? 4.2857 x 10^11 hz. the higher the frequency, the higher or lower the energy? this is an example of a an inverse or direct relationship? higher, and direct. Calculations between wavelength, frequency and energy.
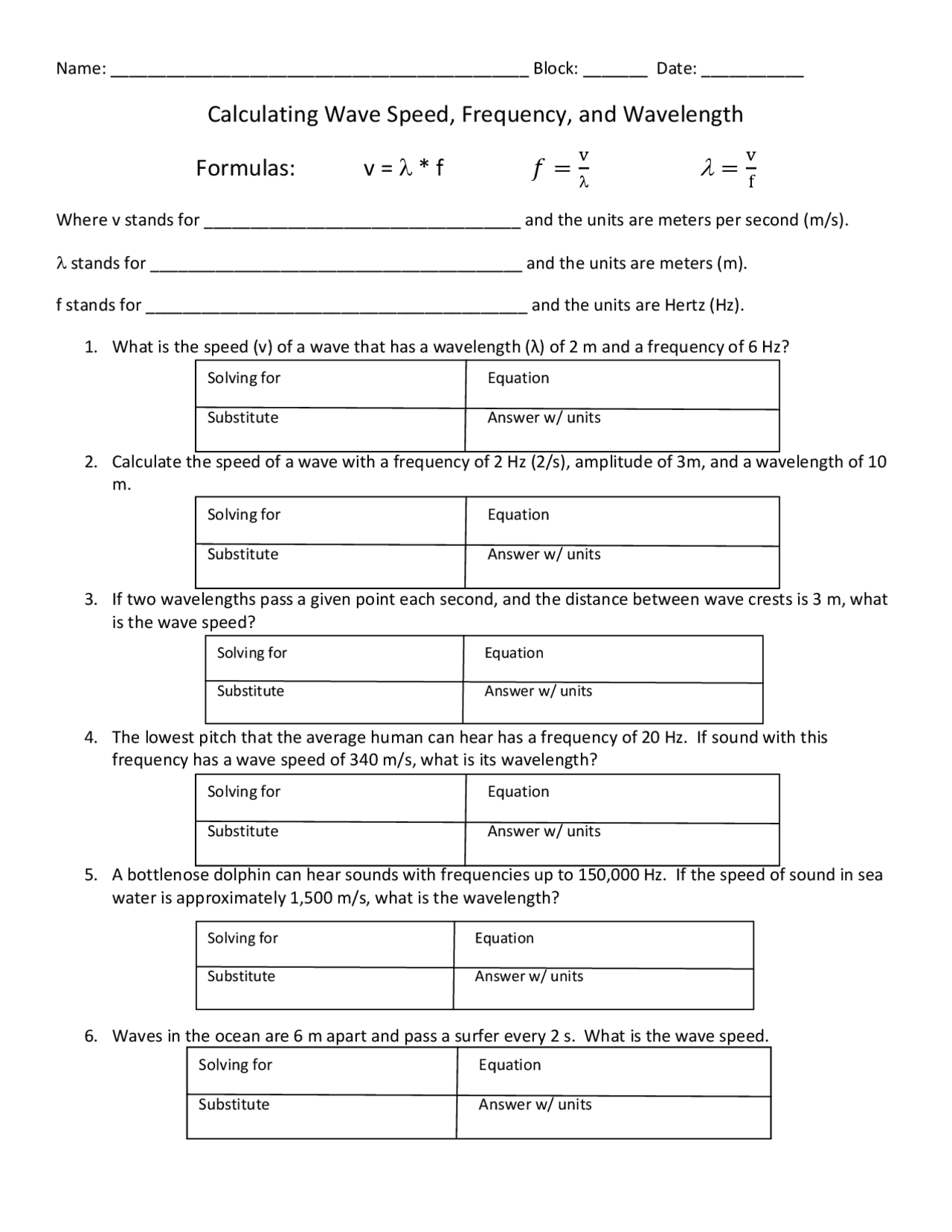
Calculating Wave Speed Frequency And Wavelength Formulas Exercises 3.0928 x 10⁻3 m. what is the frequency of a wave that has a wavelength = 97 nm? 3.09 x 10¹⁵ hz. what is the frequency of a wave that has a wavelength of 0.07 cm? 4.2857 x 10^11 hz. the higher the frequency, the higher or lower the energy? this is an example of a an inverse or direct relationship? higher, and direct. Calculations between wavelength, frequency and energy.

Comments are closed.