Ch3 Ch Ch Cho Hocl Complete The Reaction

Ch3 Ch Ch Cho Hocl Complete The Reaction Click here👆to get an answer to your question ️ b) complete the following reactions: 1) ch, ch=ch, hi. Click here👆to get an answer to your question ️ complete the reaction dch.chcecch(old, dil kmno2) ii) ch ch;bracker, iii) ch==ch2 os its.

Ch3 Ch Ch Cho Hocl Complete The Reaction Let's focus on part a first. while approaching the addition of hcl to , use the double bond present in alkene to attack the of hcl to generate a carbocation. what are the products of the following addition reactions? part a ch3—ch=ch ch3 hcl — draw the molecule (s) on the canvas by choosing buttons from the tools (for bonds), atoms, and. Aie 1 2020: the reaction of ch3 ch = ch2 with hocl will yield (a) 2 chloro 1 propanol (b) 3 chloro 2 propanol (c) 1 chloro 2 propanol (d) 1 chloro 1. The alcohol is heated under reflux with an excess of the oxidizing agent. when the reaction is complete, the carboxylic acid is distilled off. the full equation for the oxidation of ethanol to ethanoic acid is: 3ch3ch2oh 2cr2o2−7 16h → 3ch3cooh 4cr3 11h2o (12.12.2) (12.12.2) 3 c h 3 c h 2 o h 2 c r 2 o 7 2 − 16 h → 3 c h. These are examples of five different reaction types. > (i) "ch" 3"ch" 3 "cl" 2 → "ch" 3"ch" 2"cl" "hcl" this is a free radical substitution reaction. (ii) "ch.

Ch3 Ch Ch Cho Hocl Complete The Reaction The alcohol is heated under reflux with an excess of the oxidizing agent. when the reaction is complete, the carboxylic acid is distilled off. the full equation for the oxidation of ethanol to ethanoic acid is: 3ch3ch2oh 2cr2o2−7 16h → 3ch3cooh 4cr3 11h2o (12.12.2) (12.12.2) 3 c h 3 c h 2 o h 2 c r 2 o 7 2 − 16 h → 3 c h. These are examples of five different reaction types. > (i) "ch" 3"ch" 3 "cl" 2 → "ch" 3"ch" 2"cl" "hcl" this is a free radical substitution reaction. (ii) "ch. C h oh ch3cooh → ch3cooc2h3 h20 d choh o2 → co2 2h2o e ch ch2 h2 → ch ch3 c. ch2ch(oh)ch; → ch ch = ch, h2o which is true of markovnikov's rule? o d. a and b are true. a. it can be used to predict the isomer that will be most plentiful in some addition reactions. oc. Click here👆to get an answer to your question ️ ch3 ch = ch cho [ hocl ] complete the reaction.

Ch3 Ch Ch Cho Hocl Complete The Reaction C h oh ch3cooh → ch3cooc2h3 h20 d choh o2 → co2 2h2o e ch ch2 h2 → ch ch3 c. ch2ch(oh)ch; → ch ch = ch, h2o which is true of markovnikov's rule? o d. a and b are true. a. it can be used to predict the isomer that will be most plentiful in some addition reactions. oc. Click here👆to get an answer to your question ️ ch3 ch = ch cho [ hocl ] complete the reaction.
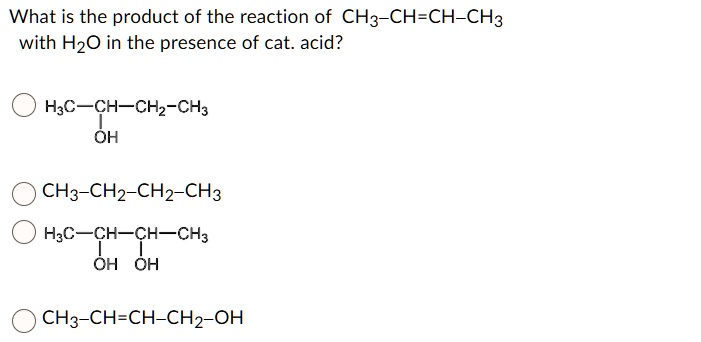
What Is The Product Of The Reaction Of Ch3 Ch Ch Ch3 вђ Solvedlib

Comments are closed.