Changes In States Of Matter Freezing Melting Condensation Evaporation Sublimation Deposition
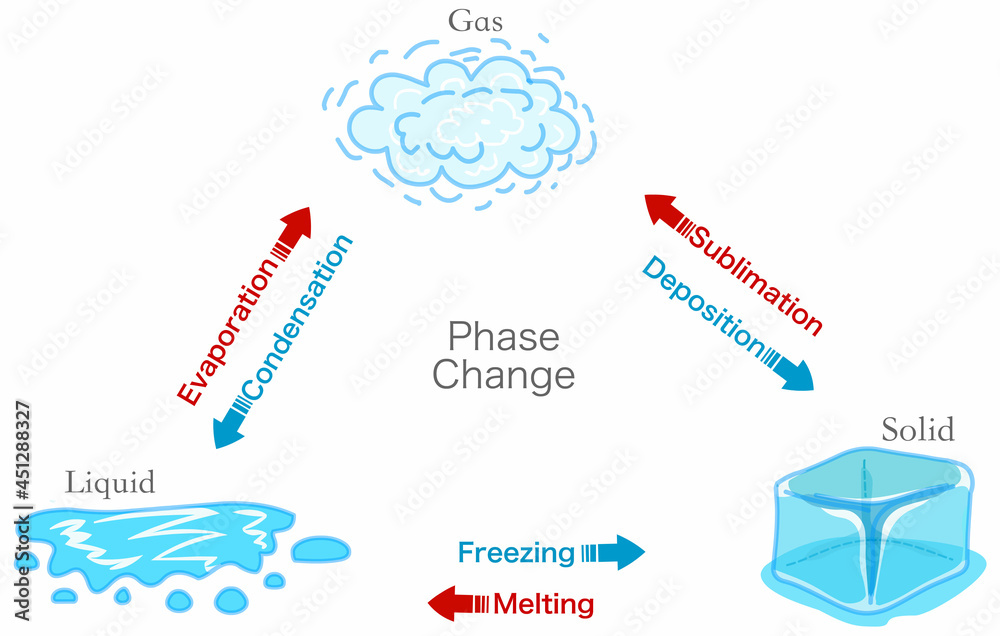
Phase Change Transition Diagram States Matter Schema Evaporation 13.3: melting, freezing, sublimation, and deposition. 5.4: phase changes.

Changes In States Of Matter Freezing Melting Condensation A phase change or phase transition is a change between solid, liquid, gaseous, and sometimes plasma states of matter. the states of matter differ in the organization of particles and their energy. the main factors that cause phase changes are changes in temperature and pressure. at the phase transition, such as the boiling point between liquid. Sublimation is the change of state from a solid to a gas, without passing through the liquid state. deposition is the change of state from a gas to a solid. carbon dioxide is an example of a material that easily undergoes sublimation. the melting point is the temperature at which a solid changes into a liquid. intermolecular forces have a. Sublimation and deposition. some solids can transition directly into the gaseous state, bypassing the liquid state, via a process known as sublimation. at room temperature and standard pressure, a piece of dry ice (solid co 2) sublimes, appearing to gradually disappear without ever forming any liquid. snow and ice sublime at temperatures below. The phase changes of matter include melting, freezing, evaporation, condensation, deposition, and sublimation.

Changing States Of Matter рџ ѓ Melting Freezing Evaporation Sublimation and deposition. some solids can transition directly into the gaseous state, bypassing the liquid state, via a process known as sublimation. at room temperature and standard pressure, a piece of dry ice (solid co 2) sublimes, appearing to gradually disappear without ever forming any liquid. snow and ice sublime at temperatures below. The phase changes of matter include melting, freezing, evaporation, condensation, deposition, and sublimation. 11.3 phase transitions. we witness and utilize changes of physical state, or phase transitions, in a great number of ways. as one example of global significance, consider the evaporation, condensation, freezing, and melting of water. these changes of state are essential aspects of our earth’s water cycle as well as many other natural. List of phase changes between states of matter.
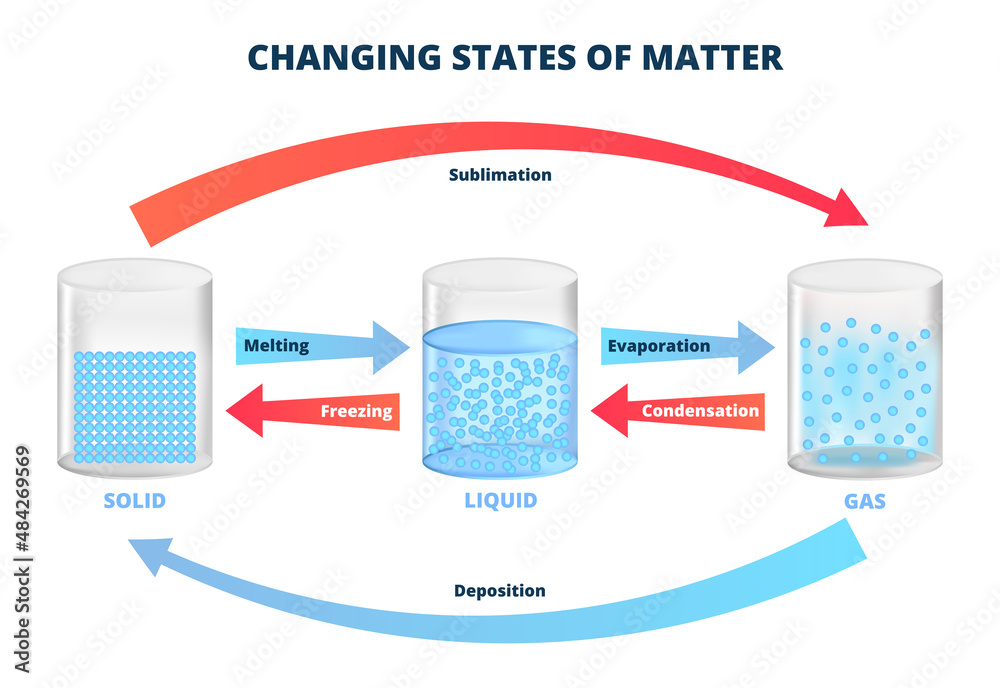
Vector Diagram With Changing States Of Matter Three States Of Matter 11.3 phase transitions. we witness and utilize changes of physical state, or phase transitions, in a great number of ways. as one example of global significance, consider the evaporation, condensation, freezing, and melting of water. these changes of state are essential aspects of our earth’s water cycle as well as many other natural. List of phase changes between states of matter.
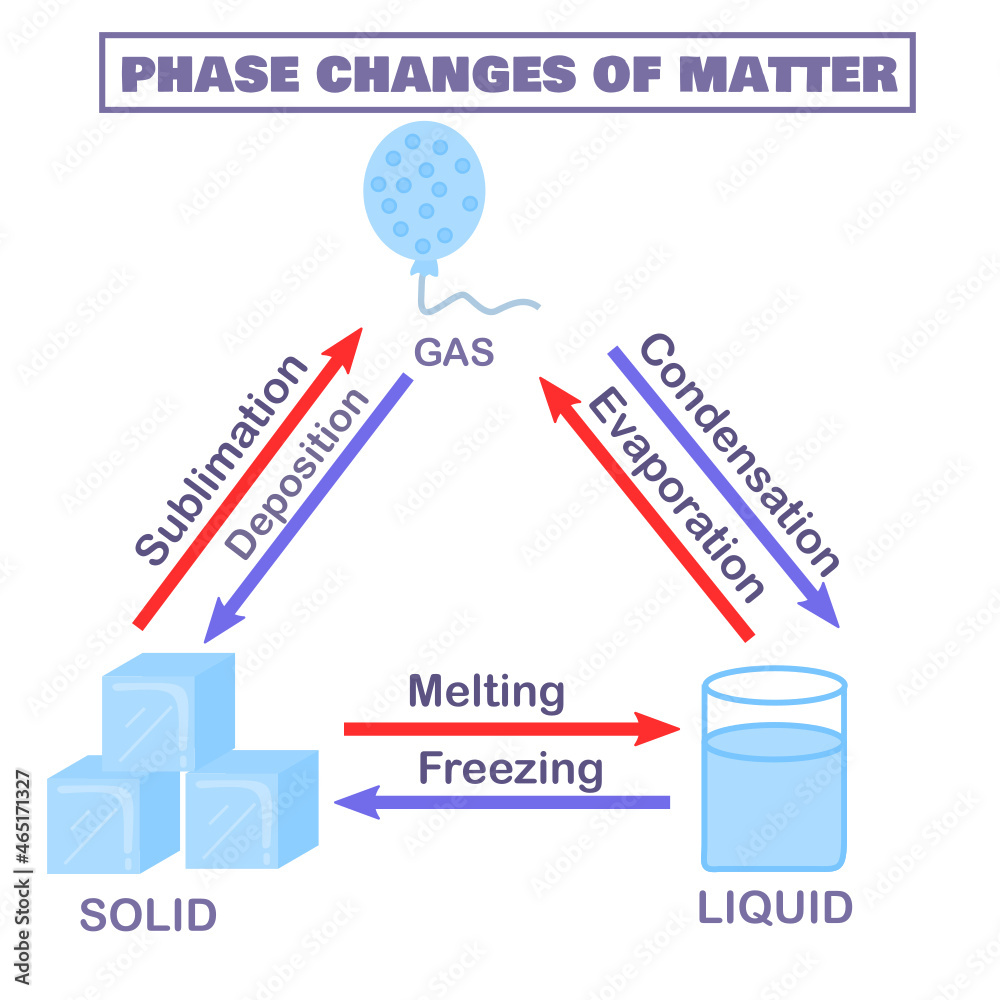
Physical States Of Matter Solid Liquid And Gas Melting Freezing

Comments are closed.