Covalent Bonding In An Oxygen Molecule

Covalent Bonding In An Oxygen Molecule An oxygen atom has 6 electrons in its outer shell. oxygen is in group 6 of the periodic table. two oxygen atoms will each share two electrons to form two covalent bonds and make an oxygen molecule (o 2). this is a picture of an oxygen molecule. by sharing the four electrons where the shells touch each oxygen atom can count 8 electrons in its. 9.2: covalent bond.
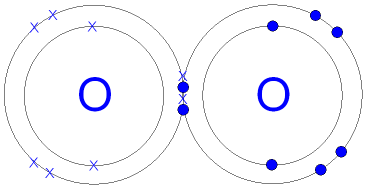
Gcse Chemistry Covalent Bonding In An Oxygen Molecule What Is The 4.2: covalent bonds. Using formal charges to distinguish viable lewis structures. 9.5: covalent bonding lewis structure. the strength of a covalent bond depends on the overlap between the valence orbitals of the bonded atoms. bond order is the number of electron pairs that hold two atoms together. single bonds have a …. The water molecule (h 2 o) is an example of compound with covalent bonds. the oxygen atom shares one electron with each of the two hydrogen atoms, forming two covalent bonds. octet rule and covalent bonding. the concept of covalent bonding ties in with the octet rule. The covalent bond. atoms can combine to achieve an octet of valence electrons by sharing electrons. two fluorine atoms, for example, can form a stable f 2 molecule in which each atom has an octet of valence electrons by sharing a pair of electrons. a pair of oxygen atoms can form an o 2 molecule in which each atom has a total of eight valence.
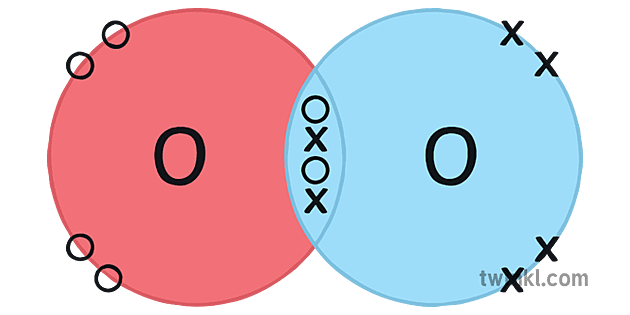
O2 Oksijeni Covalent Bonding Dot Cross Diagram Science Ks4 Ilustraciгіn The water molecule (h 2 o) is an example of compound with covalent bonds. the oxygen atom shares one electron with each of the two hydrogen atoms, forming two covalent bonds. octet rule and covalent bonding. the concept of covalent bonding ties in with the octet rule. The covalent bond. atoms can combine to achieve an octet of valence electrons by sharing electrons. two fluorine atoms, for example, can form a stable f 2 molecule in which each atom has an octet of valence electrons by sharing a pair of electrons. a pair of oxygen atoms can form an o 2 molecule in which each atom has a total of eight valence. Formation of covalent bonds. nonmetal atoms frequently form covalent bonds with other nonmetal atoms. for example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. figure 7.4 illustrates why this bond is formed. starting on the far right, we have two separate hydrogen atoms with a particular potential energy. Covalent bond covalent bond.

Covalent Bonding Ck 12 Foundation Formation of covalent bonds. nonmetal atoms frequently form covalent bonds with other nonmetal atoms. for example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. figure 7.4 illustrates why this bond is formed. starting on the far right, we have two separate hydrogen atoms with a particular potential energy. Covalent bond covalent bond.

Comments are closed.