Difference Between Case Control Study And Cohort Study

What Is The Difference Between Case Control And Cohort Study Ped Case control and cohort studies: a brief overview. Observational studies: cohort and case control studies.
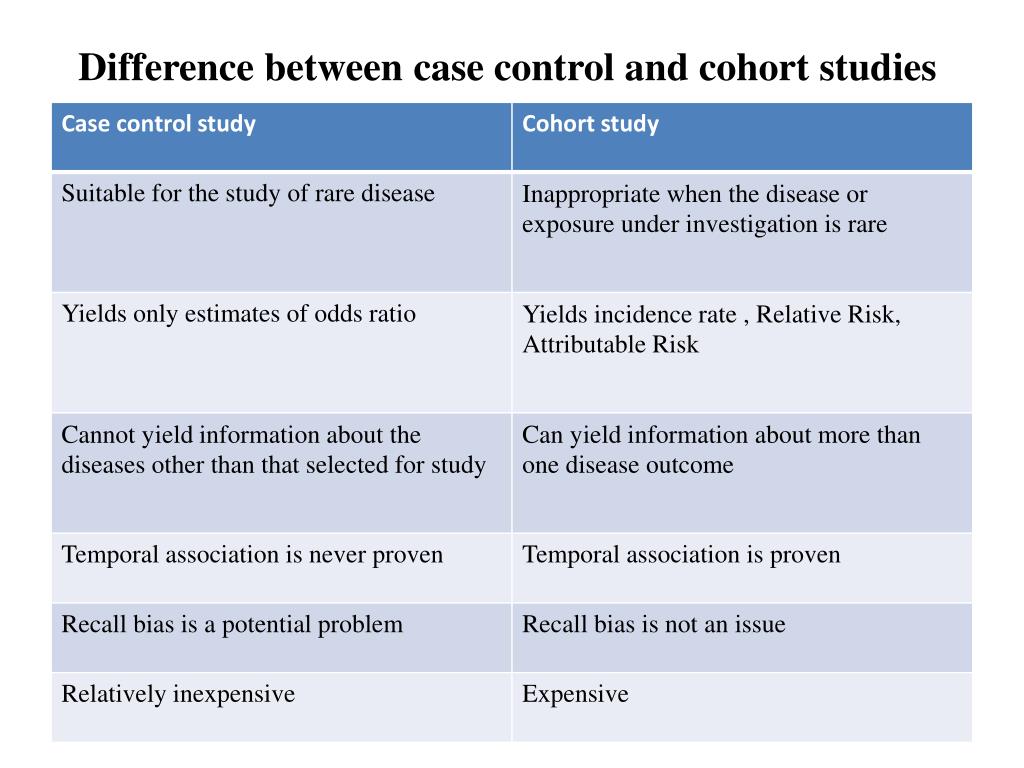
Ppt Case Control Study Powerpoint Presentation Free Download Id A case cohort study is similar to a nested case control study in that the cases and non cases are within a parent cohort; cases and non cases are identified at time t1, after baseline. in a case cohort study, the cohort members were assessed for risk factors at any time prior to t1. non cases are randomly selected from the parent cohort. For example, imagine a study of 20 consecutive patients with a certain disease that can be treated in two different ways. a study that divides the 20 patients into two groups according to the treatment received and compares the outcomes of these groups (e.g., provides aggregated absolute risks per group or a risk ratio) would be probably classified as a cohort study (the example used in the. In a case–control study, a number of cases and noncases (controls) are identified, and the occurrence of one or more prior exposures is compared between groups to evaluate drug–outcome associations (figure 1). a case–control study runs in reverse relative to a cohort study. 21 as such, study inception occurs when a patient experiences an. Case control studies and cohort studies are two common types of observational studies used in epidemiology to investigate the association between exposure and disease outcomes. in a case control study, researchers start by identifying individuals with the disease (cases) and individuals without the disease (controls) and then retrospectively.
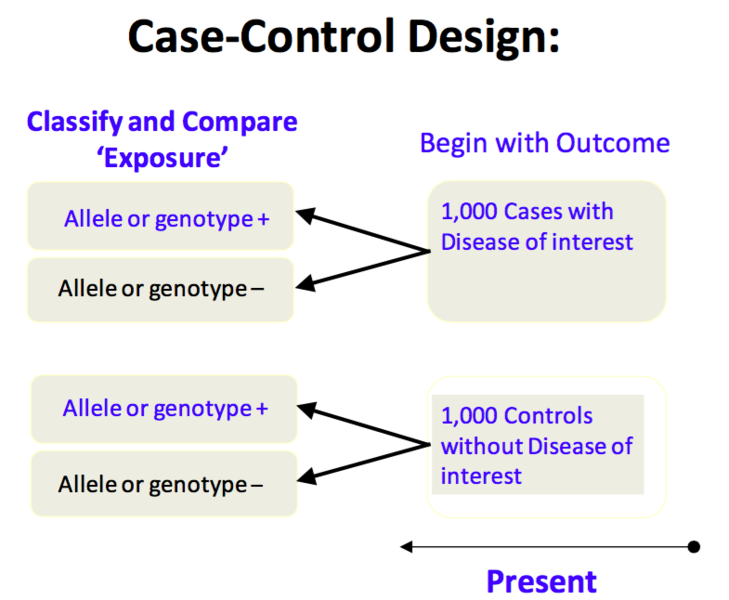
What Is The Difference Between Case Control And Cohort Study Ped In a case–control study, a number of cases and noncases (controls) are identified, and the occurrence of one or more prior exposures is compared between groups to evaluate drug–outcome associations (figure 1). a case–control study runs in reverse relative to a cohort study. 21 as such, study inception occurs when a patient experiences an. Case control studies and cohort studies are two common types of observational studies used in epidemiology to investigate the association between exposure and disease outcomes. in a case control study, researchers start by identifying individuals with the disease (cases) and individuals without the disease (controls) and then retrospectively. A case control study differs from a cohort study because cohort studies are more longitudinal in nature and do not necessarily require a control group. while one may be added if the investigator so chooses, members of the cohort are primarily selected because of a shared characteristic among them. in particular, retrospective cohort studies are. Revised on june 22, 2023. a case control study is an experimental design that compares a group of participants possessing a condition of interest to a very similar group lacking that condition. here, the participants possessing the attribute of study, such as a disease, are called the “case,” and those without it are the “control.”.

Comments are closed.