Explain A Neutralization Reaction

Neutralization Reaction Definition And Products A neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. in a neutralization reaction, there is a combination of h ions and oh – ions which form water. a neutralisation reaction is generally an acid base neutralization reaction. A neutralization reaction is a chemical reaction between an acid and a base that forms a salt and water as products. in other words, the reaction neutralizes the acid and base. a neutralization reaction is a type of double replacement reaction in which the reactants are an acid and a base. the acid releases a hydrogen ion in water, while the.
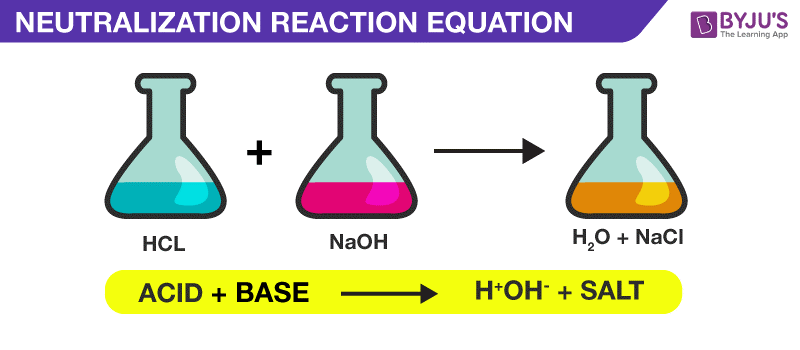
Neutralization Reaction Definition Examples Applications Weak acid weak base neutralization. a weak acid, weak base reaction can be shown by the net ionic equation example: \(h^ {(aq)} nh {3(aq)} \leftrightharpoons nh^ {4 (aq)} \) the equivalence point of a neutralization reaction is when both the acid and the base in the reaction have been completely consumed and neither of them are in excess. False. neutralisation reactions are reversible. give an example of neutralisation in day to day life. medicines such as anti acids contain aluminium hydroxide and magnesium hydroxide to neutralise the excess acid in the stomach. briefly explain why the reaction between acid and base is termed a neutralisation reaction?. The reaction of an acid and a base is called a neutralization reaction. although acids and bases have their own unique chemistries, the acid and base cancel each other's chemistry to produce a rather innocuous substance—water. in fact, the general reaction between an acid and a base is: acid base → water salt acid base → water salt. Neutralization (chemistry) animation of a strong acid–strong base neutralization titration (using phenolphthalein ). the equivalence point is marked in red. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity of each other.

Neutralization Reaction Youtube The reaction of an acid and a base is called a neutralization reaction. although acids and bases have their own unique chemistries, the acid and base cancel each other's chemistry to produce a rather innocuous substance—water. in fact, the general reaction between an acid and a base is: acid base → water salt acid base → water salt. Neutralization (chemistry) animation of a strong acid–strong base neutralization titration (using phenolphthalein ). the equivalence point is marked in red. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react with an equivalent quantity of each other. Neutralization reactions and net ionic equations for neutralization reactions. a neutralization reaction is a reaction in which an acid and a base react in an aqueous solution to produce a salt and water. the aqueous sodium chloride that is produced in the reaction is called a salt. Write the complete and net ionic equations for the neutralization reaction between h 2 c 2 o 4 (s) and sr(oh) 2 (aq). assume the salt is insoluble. explain why the net ionic equation for the neutralization reaction between hcl(aq) and koh(aq) is the same as the net ionic equation for the neutralization reaction between hno 3 (aq) and rboh.

What Is Neutralisation In Chemistry The Chemistry Blog Neutralization reactions and net ionic equations for neutralization reactions. a neutralization reaction is a reaction in which an acid and a base react in an aqueous solution to produce a salt and water. the aqueous sodium chloride that is produced in the reaction is called a salt. Write the complete and net ionic equations for the neutralization reaction between h 2 c 2 o 4 (s) and sr(oh) 2 (aq). assume the salt is insoluble. explain why the net ionic equation for the neutralization reaction between hcl(aq) and koh(aq) is the same as the net ionic equation for the neutralization reaction between hno 3 (aq) and rboh.

Neutralization Reaction Definition Equation And Examples Teachoo

Comments are closed.