Explained Classifying Matter Elements Compounds Mixture
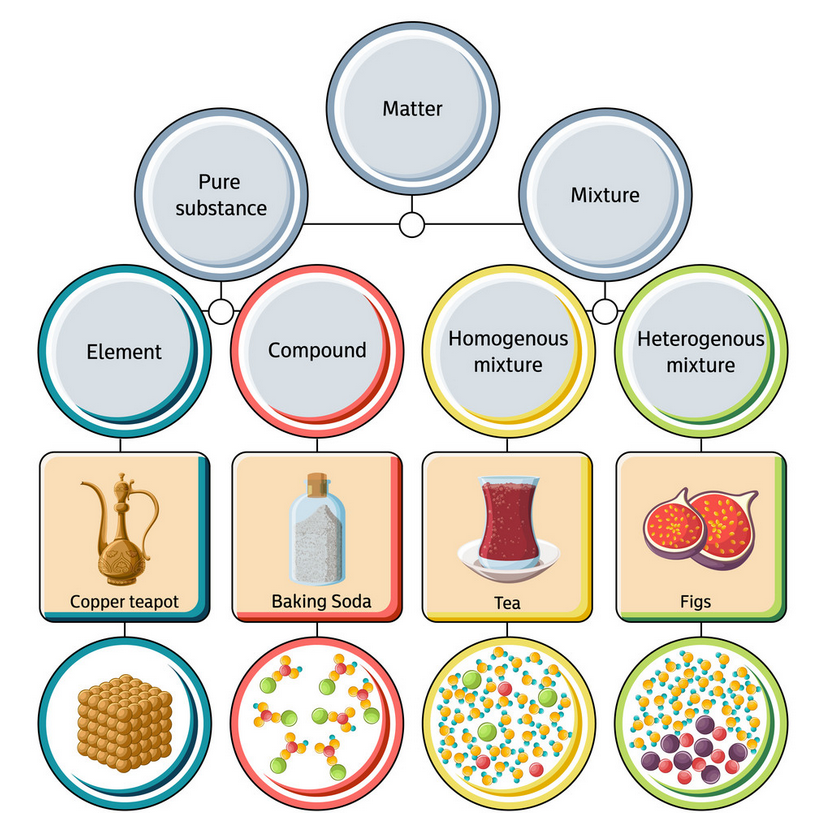
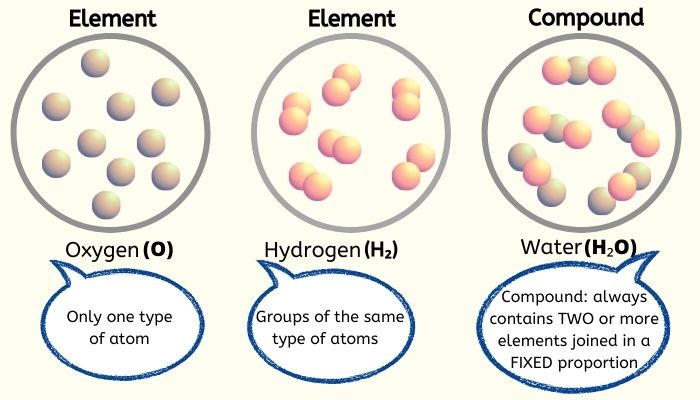
Classifying Matter A compound is a pure substance that contains two or more types of atoms or elements that are chemically joined together in a fixed proportion. compounds can be broken down into elements through chemical change. a chemical change is a change that forms one or more new substances. for example, carbon dioxide (co2) is also a compound. 19 classification of matter (elements, compounds, mixtures).
Explained Classifying Matter Elements Compounds Mixture This chemistry video tutorial provides a basic introduction into the different types of matter such as elements, compounds, mixtures, and pure substances.che. Figure 1.2.3 1.2. 3: the three most common states or phases of matter are solid, liquid, and gas. (cc by 4.0; openstax) a beaker labeled solid contains a cube of red matter and says has fixed shape and volume. a beaker labeled liquid contains a brownish red colored liquid. Figure 3.4.1 3.4. 1: relationships between the types of matter and the methods used to separate mixtures. ordinary table salt is called sodium chloride. it is considered a substance because it has a uniform and definite composition. all samples of sodium chloride are chemically identical. water is also a pure substance. Matter can be classified according to physical and chemical properties. matter is anything that occupies space and has mass. the three states of matter are solid, liquid, and gas. a physical change involves the conversion of a substance from one state of matter to another, without changing its chemical composition.
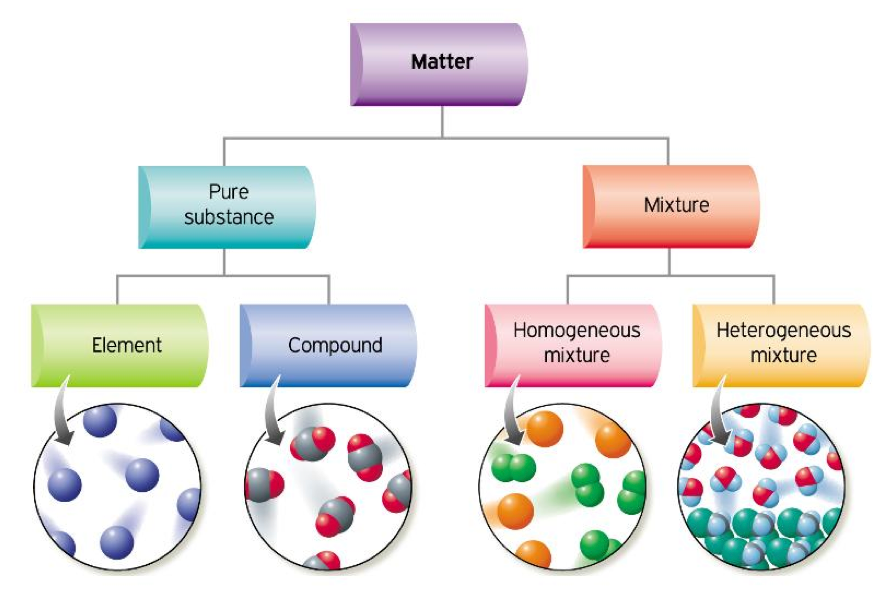
Diagram Of Mixtures Elements And Compounds Figure 3.4.1 3.4. 1: relationships between the types of matter and the methods used to separate mixtures. ordinary table salt is called sodium chloride. it is considered a substance because it has a uniform and definite composition. all samples of sodium chloride are chemically identical. water is also a pure substance. Matter can be classified according to physical and chemical properties. matter is anything that occupies space and has mass. the three states of matter are solid, liquid, and gas. a physical change involves the conversion of a substance from one state of matter to another, without changing its chemical composition. Pure substances that are comprised of two or more elements are called compounds. compounds may be broken down by chemical changes to yield either elements or other compounds, or both. for example, mercury (ii) oxide, an orange, crystalline solid, can be broken down by heat into the elements mercury and oxygen (figure 4). Classifying matter. we can classify matter into several categories. two broad categories are mixtures and pure substances. a pure substance has a constant composition. all specimens of a pure substance have exactly the same makeup and properties. any sample of sucrose (table sugar) consists of 42.1% carbon, 6.5% hydrogen, and 51.4% oxygen by mass.

Comments are closed.