Functional Group Priorities For Naming Organic Compounds With Multiple Functional Functional
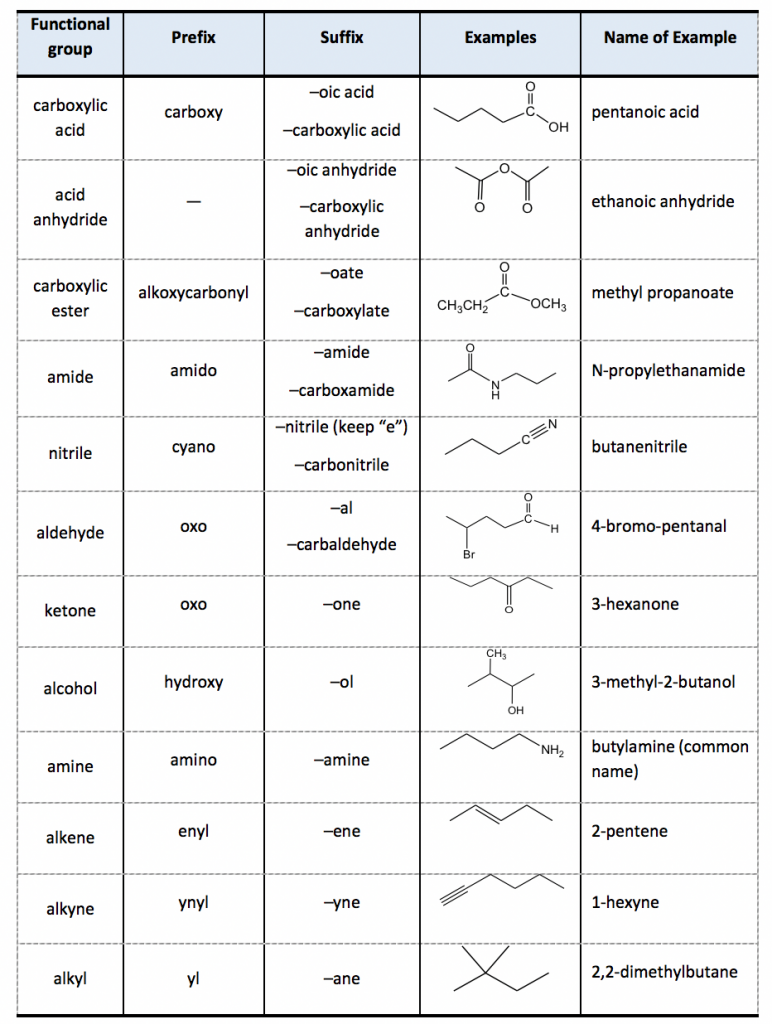
2 4 Iupac Naming Of Organic Compounds With Functional Groups вђ Organicођ He functional group with the highest priority will be the one which gives its suffix to the name of the molecule. so in example #1 above, the suffix of the molecule will be “ oic acid” , not “ one”, because carboxylic acids are given higher priority. however, if a ketone is present with an alcohol (example 3) then we will use the suffix. For naming purposes, the functional groups are assigned with priorities (table 2.3). if the compound includes more than one functional groups, the one with the highest priority is the “parent structure” and determines the “parent name”; the other groups will be regarded as “substituents”. “suffix” is used to indicate the name of.

Table Of Functional Group Priorities For Nomenclature Organic Step 3. number the parent chain starting from the highest priority group and add the substituent (s) alphabetically: it is also noteworthy that if there is a functional group suffix and a substituent, the functional group suffix gets the lowest possible number. for example, alcohols have higher priority than amines and therefore, when naming a. In the iupac nomenclature system, organic molecules are grouped into specific classes of compounds determined by the main functional group present in the structure. a system of priorities is used to determine the main functional group, which determines the identity of the compound. all other functional groups are treated as substituents. Identify the functional groups (other than alkanes) in the following organic compounds. state whether alcohols and amines are primary, secondary, or tertiary. solutions to exercises. a) carboxylate, sulfide, aromatic, two amide groups (one of which is cyclic) b) tertiary alcohol, thioester. c) carboxylate, ketone. Organic chemistry functional groups: a complete guide to recognizing, drawing, and naming ( priority cheat sheet at bottom) a functional group is a specific group of atoms that helps determine the chemistry and reactivity of the overall molecule. when classifying functional groups, we look at both the specific atoms present, as well as the.
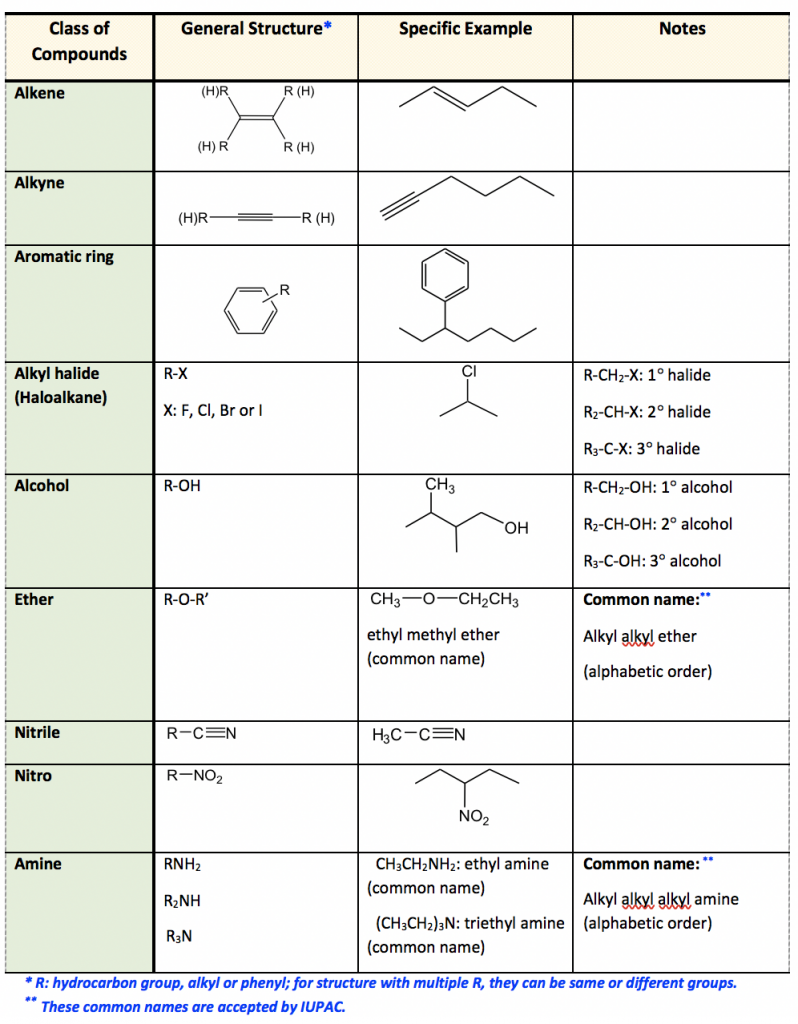
2 3 Functional Groups вђ Organic Chemistry I Identify the functional groups (other than alkanes) in the following organic compounds. state whether alcohols and amines are primary, secondary, or tertiary. solutions to exercises. a) carboxylate, sulfide, aromatic, two amide groups (one of which is cyclic) b) tertiary alcohol, thioester. c) carboxylate, ketone. Organic chemistry functional groups: a complete guide to recognizing, drawing, and naming ( priority cheat sheet at bottom) a functional group is a specific group of atoms that helps determine the chemistry and reactivity of the overall molecule. when classifying functional groups, we look at both the specific atoms present, as well as the. This systematic nomenclature arrives at the compound name in the following order: step 1 longest carbon chain. step 2 unsaturation. step 3 functional groups. step 4 position of any functional groups. step 1: find the longest unbroken carbon chain and use this as the basis for the root name of the compound. number of carbon atoms. root. Hence, the iupac name will be 6 methyloctan 3 ol. name the functional group given below: this compound has the functional group as ketone (>c=o). hence, the suffix will be one. and there are two ketone groups. so we will use di before suffix as dione. continuing in the same manner as above we get the name hexane 2, 4 dione. in this article.
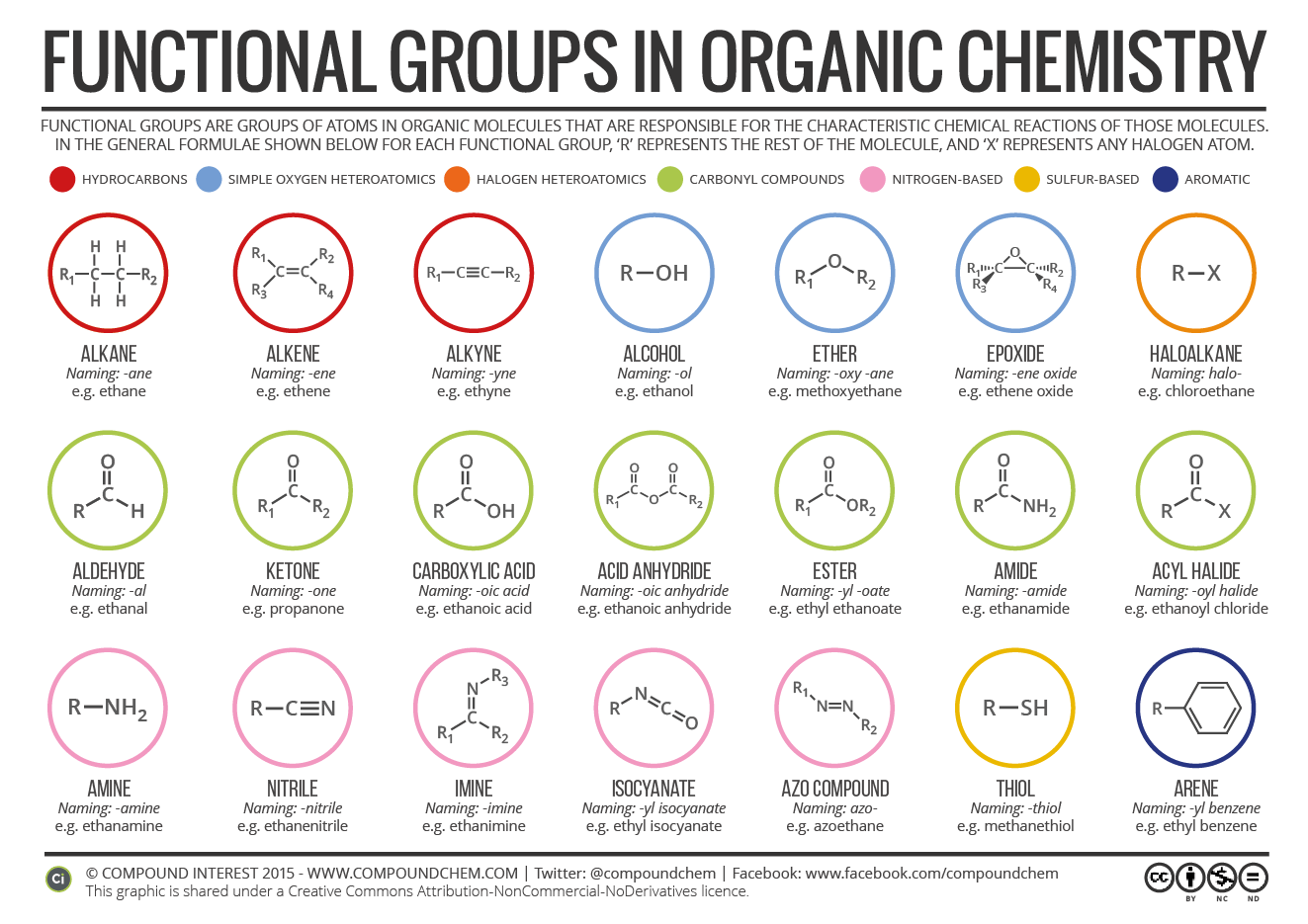
Functional Groups In Organic Compounds Compound Interest This systematic nomenclature arrives at the compound name in the following order: step 1 longest carbon chain. step 2 unsaturation. step 3 functional groups. step 4 position of any functional groups. step 1: find the longest unbroken carbon chain and use this as the basis for the root name of the compound. number of carbon atoms. root. Hence, the iupac name will be 6 methyloctan 3 ol. name the functional group given below: this compound has the functional group as ketone (>c=o). hence, the suffix will be one. and there are two ketone groups. so we will use di before suffix as dione. continuing in the same manner as above we get the name hexane 2, 4 dione. in this article.

Comments are closed.