Heat Transfer Units

Heat Transfer Units Learn about the three types of heat transfer: conduction, convection, and radiation. find out how they differ, what they involve, and how to calculate them with equations. Learn about the four fundamental modes of heat transfer: advection, conduction, convection, and radiation. find out how heat transfer is involved in various engineering fields and processes, such as thermal hydraulics, phase changes, and fourier's law.

Heating Compact Unit Compact Heat Transfer Unit Ecoamigo Macau Hk Heat transfer is a process in which the thermal energy of molecules is moved from the region of a higher temperature to a lower temperature. the heat transfer unit is usually the joule, or in the case of heat transfer per unit time, it is watts or kilocalories per second. Learn about the proportionality constant between heat flux and temperature difference, and how to calculate it for different modes, fluids and conditions. find formulas, examples and online calculators for convective heat transfer correlations. This realization helped establish that heat is a form of energy. james prescott joule (1818–1889) performed many experiments to establish the mechanical equivalent of heat —the work needed to produce the same effects as heat transfer. in the units used for these two quantities, the value for this equivalence is \[1.000 \, kcal = 4186 \, j.\]. The unit that remains after these cancelations is "cal," which, per the information in the given problem, is the unit in which the unknown quantity, heat transfer, q, must be expressed. applying the correct number of significant figures to the calculated quantity results in the final answer that is shown below.

Ht10x Computer Controlled Heat Transfer Service Unit Armfield This realization helped establish that heat is a form of energy. james prescott joule (1818–1889) performed many experiments to establish the mechanical equivalent of heat —the work needed to produce the same effects as heat transfer. in the units used for these two quantities, the value for this equivalence is \[1.000 \, kcal = 4186 \, j.\]. The unit that remains after these cancelations is "cal," which, per the information in the given problem, is the unit in which the unknown quantity, heat transfer, q, must be expressed. applying the correct number of significant figures to the calculated quantity results in the final answer that is shown below. Learn the basic terms and concepts of heat transfer, such as heat, temperature, conduction, convection, radiation, and heat exchangers. find definitions, formulas, and examples of heat transfer units and properties. Solution. use the equation for heat transfer q = mcΔt. q = m c Δ t. q = m c Δ t to express the heat transferred from the pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan, and the final temperature: qhot = ma1ca1 (tf − 150°c). q hot = m a1 c a1 (t f − 150 ° c).
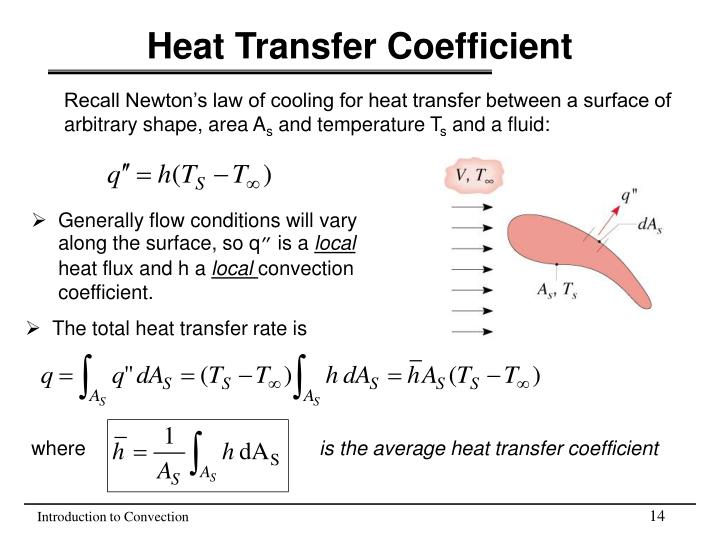
Convective Heat Transfer Coefficient Units Learn the basic terms and concepts of heat transfer, such as heat, temperature, conduction, convection, radiation, and heat exchangers. find definitions, formulas, and examples of heat transfer units and properties. Solution. use the equation for heat transfer q = mcΔt. q = m c Δ t. q = m c Δ t to express the heat transferred from the pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan, and the final temperature: qhot = ma1ca1 (tf − 150°c). q hot = m a1 c a1 (t f − 150 ° c).

Comments are closed.