How To Calculate Equilibrium Concentrations From Equilibrium Constant
How To Calculate Equilibrium Concentrations From Equilibrium Constant 15.5: calculating equilibrium constants. Calculating equilibrium concentrations from the equilibrium constant. to describe how to calculate equilibrium concentrations from an equilibrium constant, we will again consider a system that contains only a single product and a single reactant, the conversion of n butane to isobutane (equation \(\ref{eq1}\)), for which k = 2.6 at 25°c.
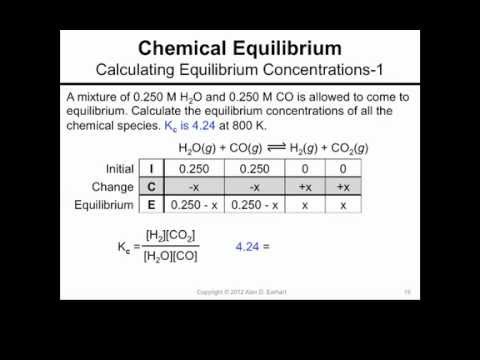
Calculating Equilibrium Concentrations 1 Youtube 15.2: the equilibrium constant (k). Example #1: calculate the equilibrium constant (k c) for the following reaction: h 2 (g) i 2 (g) ⇌ 2hi (g) when the equilibrium concentrations at 25.0 °c were found to be: [h 2] = 0.0505 m [i 2] = 0.0498 m [hi] = 0.389 m. solution: 1) the first thing to do is write the equilibrium expression for the reaction as written in the problem. Calculate values of reaction quotients and equilibrium constants, using concentrations and pressures relate the magnitude of an equilibrium constant to properties of the chemical system the status of a reversible reaction is conveniently assessed by evaluating its reaction quotient ( q ) . Calculating equilibrium constants. we need to know two things in order to calculate the numeric value of the equilibrium constant: the balanced equation for the reaction system, including the physical states of each species. from this the equilibrium expression for calculating k c or k p is derived. the equilibrium concentrations or pressures.
How To Calculate The Equilibrium Constant Haiper Calculate values of reaction quotients and equilibrium constants, using concentrations and pressures relate the magnitude of an equilibrium constant to properties of the chemical system the status of a reversible reaction is conveniently assessed by evaluating its reaction quotient ( q ) . Calculating equilibrium constants. we need to know two things in order to calculate the numeric value of the equilibrium constant: the balanced equation for the reaction system, including the physical states of each species. from this the equilibrium expression for calculating k c or k p is derived. the equilibrium concentrations or pressures. Nitrogen oxides are air pollutants produced by the reaction of nitrogen and oxygen at high temperatures. at 2000 °c, the value of the equilibrium constant for the reaction, n2(g) o2(g) ⇌ 2no(g) n 2 (g) o 2 (g) ⇌ 2 no (g), is 4.1 × 10 −4. find the concentration of no no (g) in an equilibrium mixture with air at 1 atm pressure at this. Physical chemistry (essentials) class 11 8 units · 52 skills. unit 1 welcome to physical chemistry. unit 2 structure of atom. unit 3 some basic concepts of chemistry. unit 4 redox reactions. unit 5 gaseous state. unit 6 thermodynamics. unit 7 chemical equilibrium. unit 8 ionic equilibrium.

Comments are closed.