How To Draw Diagram To Convert Between Reference Electrodes
Solved 15 2 Draw A Diagram Like Figure 15 6 To Convert The Following Figure 5: electrode potentials vs she for the two reference electrodes in the dataset using \(\ce{kcl(aq)}\) electrolyte (each panel shows one reference electrode, each line shows a different electrolyte concentration). just to demonstrate the extent of the dataset in parameter space. 23.1: reference electrodes. in potentiometry we measure the difference between the potential of two electrodes. the potential of one electrode—the working or indicator electrode—responds to the analyte’s activity and the other electrode—the counter or reference electrode—has a known, fixed potential. by convention, the reference.

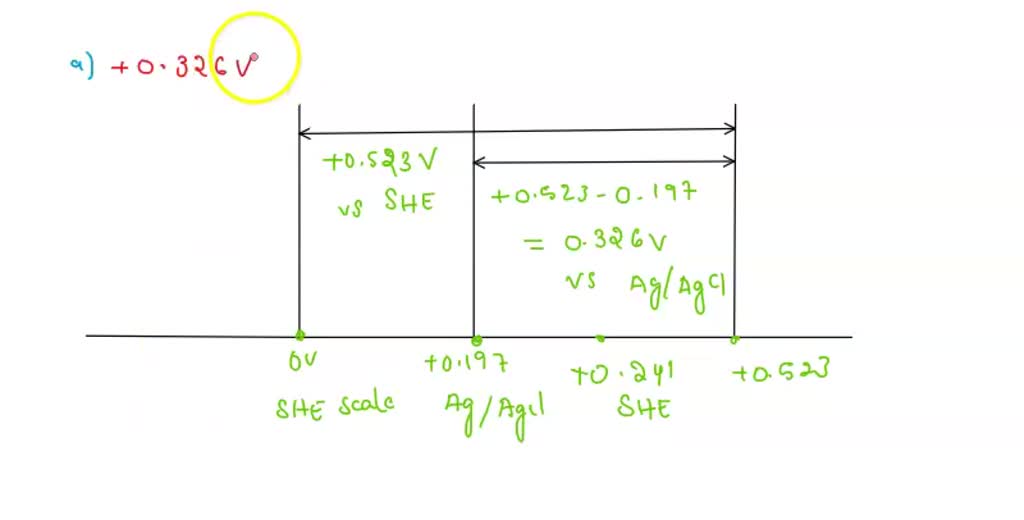
Conversion Between Reference Electrodes Youtube In many electrodes designed for potentiometry, the reference half cell is contained within the body of the sensing electrode. this arrangement is referred to as a “combination” electrode. 2. silver silver chloride (ag agcl) the silver silver chloride reference electrode is composed of a silver wire, sometimes coated with a layer of solid. If you click 'convert' on the calculator below, the measured potential is marked up on the scale and we can see that for the above problem the converted potential is 0.144 v (vs ag agcl sat.) i.e. 0.1 v 0.241 v 0.197 v = 0.144 v. just be aware that the reported electrode values for different systems can vary a little in the literature. A reference electrode is an electrode that has a stable and well known electrode potential. the overall chemical reaction taking place in a cell is made up of two independent half reactions, which describe chemical changes at the two electrodes. to focus on the reaction at the working electrode, the reference electrode is standardized with. Convert potentials to another reference electrode. enter the recorded voltage in mv and select the reference electrode used to record it. *calculations derived from "electrochemical methods: fundamentals and applications", a j bard and l r faulkner, john wiley & sons, ny (2000). isbn 0471405213.

Chemical World Electro Chemistry A reference electrode is an electrode that has a stable and well known electrode potential. the overall chemical reaction taking place in a cell is made up of two independent half reactions, which describe chemical changes at the two electrodes. to focus on the reaction at the working electrode, the reference electrode is standardized with. Convert potentials to another reference electrode. enter the recorded voltage in mv and select the reference electrode used to record it. *calculations derived from "electrochemical methods: fundamentals and applications", a j bard and l r faulkner, john wiley & sons, ny (2000). isbn 0471405213. The conversion equations for the reference electrodes listed in table 1 are: escesat = eshe−0.2415 e s c e s a t = e s h e − 0.2415. esscsat = eshe−0.2224 e s s c s a t = e s h e − 0.2224. emmssat = eshe−0.6150 e m m s s a t = e s h e − 0.6150. eccssat = eshe−0.3180 e c c s s a t = e s h e − 0.3180. to convert between scales we. From the little graphic, above, you should report the voltage of electrode x as 0.0v vs. ag agcl or 0.045v vs. sce. so, the short answer is “you should subtract 45 mv from readings obtained with an ag agcl,sat’d nacl reference electrode in order to convert to potential vs. sce. you may wish to think of it as adding 45 mv, since this is.

Comments are closed.