How To Use Ide Ite Ate Hypo Per Bi Di Ous And Ic Naming Ions And Ionic Compounds In

How To Use Ide Ite Ate Hypo Per Bi Di Ous A Naming ionic compounds using ite and ate. some polyatomic anions contain oxygen. these anions are called oxyanions. when an element forms two oxyanions, the one with less oxygen is given a name ending in ite and the one with more oxygen are given a name that ends in ate. no 2 nitrite. Neet. about press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket.
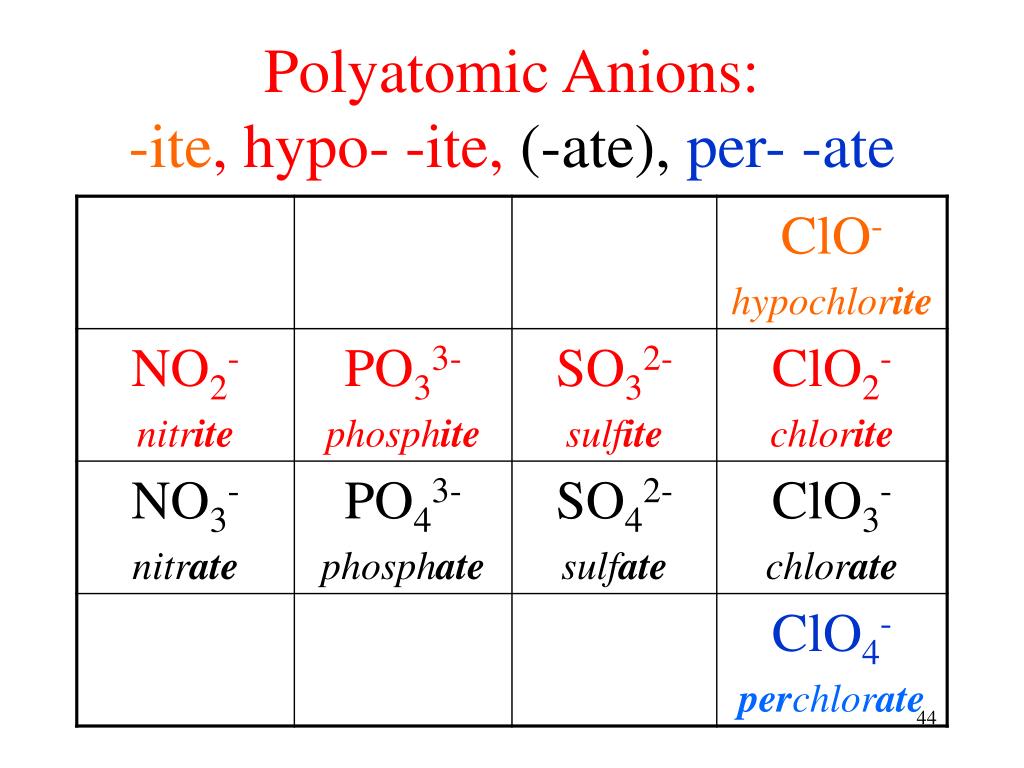
Ppt Molecules And Compounds Nomenclature Powerpoint Presentation The name of an ionic compound close ionic compound an ionic compound occurs when a negative ion (an atom that has gained an electron) joins with a positive ion (an atom that has lost an electron. Prefix and suffix based naming of inorganic compounds, ide, ite , ate, bi , di , ous, ic, hypo , per , acid naming, ortho , meta , pyro. In particular, oxyanions. basically, when you have an anion that is a combination of a non metal with oxygen. according to my book: it ends with ate for the most common oxyanions of the element. it ends with ite for the oxyanions that have the same charge but with one less atom of oxygen. example:. The name of the ion usually ends in either ite or ate. the ite ending indicates a low oxidation state. thus,the no 2 ion is the nitrite ion. the ate ending indicates a high oxidation state. the no 3 ion, for example, is the nitrate ion. the prefix hypo is used to indicate the very lowest oxidation state. the clo ion, for example, is the.

How To Use Ite Ide Ate Hypo Per Bi Di Ous A In particular, oxyanions. basically, when you have an anion that is a combination of a non metal with oxygen. according to my book: it ends with ate for the most common oxyanions of the element. it ends with ite for the oxyanions that have the same charge but with one less atom of oxygen. example:. The name of the ion usually ends in either ite or ate. the ite ending indicates a low oxidation state. thus,the no 2 ion is the nitrite ion. the ate ending indicates a high oxidation state. the no 3 ion, for example, is the nitrate ion. the prefix hypo is used to indicate the very lowest oxidation state. the clo ion, for example, is the. Hcl (g) = hydrogen chlor ide > hcl (aq) = hydro chlor ic acid. h 2 s (g) = hydrogen sulf ide > h 2 s (aq) = hydro sulfur ic acid. it is very important to include (aq) after the acids because the same compounds can be written in gas phase with hydrogen named first followed by the anion ending with –ide. example 5. 2.7: nomenclature.

How To Use Ide Ite Ate Hypo Per Inorganic Nomenclature Youtube Hcl (g) = hydrogen chlor ide > hcl (aq) = hydro chlor ic acid. h 2 s (g) = hydrogen sulf ide > h 2 s (aq) = hydro sulfur ic acid. it is very important to include (aq) after the acids because the same compounds can be written in gas phase with hydrogen named first followed by the anion ending with –ide. example 5. 2.7: nomenclature.

Comments are closed.