Ion Selective Electrode Principle Advantages Disadvantages

Ion Selective Electrode Principle Advantages Disadvantages Ion selective electrodes can be used: to determine various ions in aqueous solutions. to measure the ph of the solution. to monitor pollutants in natural water and effluents by measuring cn –, f –, s –, cl –, no3 – etc. to measure different types of ions present in the soil. to measure no3 –, no2 – ions in meat preservatives, and. Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. ise has many advantages compared to other techniques, including: it is relatively inexpensive and easy to operate. it has wide concentration measurement range.
Ion Selective Electrode Ise Principle Types Advantages And Figure 1. electrochemical cell for making a potentiometric measurement with an ise. as indicated by their name, ion selective electrodes possess a high degree of selectivity. the selectivity of the ise is determined by the composition of the membrane. ideally the membrane allows the uptake of only one specific ion into it. Ion selective electrode. Ion selective electrode (ise) analysis is an electroanalytical technique that uses an electrochemical cell consisting of two electrodes and a sample solution (electrolyte). the electrodes contain an ion permissible membrane that is selective of the ion that passes through it. this membrane separates the sample from the electrode internals. The design of a ph electrode will be discussed in detail and will exemplify the method by which other ion selective electrodes function. 7.1.1. ph electrode the components necessary for the measurement of ph involve internal and external ag agcl reference electrodes and a thin glass membrane (about 50 um thick) (figure 9).
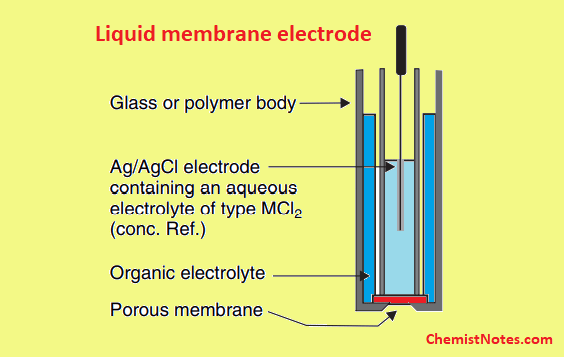
Ion Selective Electrode Ise Principle Types Advantages And Ion selective electrode (ise) analysis is an electroanalytical technique that uses an electrochemical cell consisting of two electrodes and a sample solution (electrolyte). the electrodes contain an ion permissible membrane that is selective of the ion that passes through it. this membrane separates the sample from the electrode internals. The design of a ph electrode will be discussed in detail and will exemplify the method by which other ion selective electrodes function. 7.1.1. ph electrode the components necessary for the measurement of ph involve internal and external ag agcl reference electrodes and a thin glass membrane (about 50 um thick) (figure 9). Ion selective electrode ( ch 15 4 to 15 6) • to describe the principle of the i.s.e. and its advantages and limitations (do ex. 15 32 & 15 37) • to describe the four types of i.s.e. (do ex. 15 27) • to describe the working principle of the glass electrode and perform related calculations (do ex. 15 26). Ion selective electrode (ise) is an analytical technique used to determine the activity of ions in aqueous solution by measuring the electrical potential. ise has many advantages compared to other techniques, including: it is relatively inexpensive and easy to operate. it has wide concentration measurement range.

Comments are closed.