Irreversible Vs Reversible Cooling Phase Diagrams

Comparison Between P V Diagrams For Reversible And Irreversible 1. figure 4.2.1 4.2. 1: a gas expanding from half of a container to the entire container (a) before and (b) after the wall in the middle is removed. because half of the container is under vacuum before the gas expands there, we do not expect any work to be done by the system—that is, w = 0 w = 0 because no force from the vacuum is exerted. Phenomenon associated with a natural process. irreversible process. process in which neither the system nor its environment can be restored to their original states at the same time. reversible process. process in which both the system and the external environment theoretically can be returned to their original states.
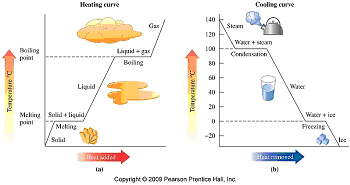
Chemistry Unit Two Matter And Energy Temperature And Kinetics Three heating–cooling treatment cycles near the phase boundary were conducted. it is found that the first cycle is partially recov erable, whereas the next two (second and third) cycles are fully recoverable. below the critical concentration (60 wt% peo), the distinct irreversible phase transition behavior with a large heat loss ionic liquids. Another example of irreversible change is the conversion of mechanical work into frictional heat; there is no way, by reversing the motion of a weight along a surface, that the heat released due to friction can be restored to the system. figure \(\pageindex{1}\): reversible vs. irreversible expansions and compressions. (cc by nc; Ümit kaya). Notice from the above diagram showing the two heat engines that for an irreversible engine having the same value of heat transfer from the thermal source q h as the reversible engine, the heat transfer to the thermal sink q l,irrev > q l,rev. let q diff = (q l,irrev – q l,rev), then the cyclic integral for an irreversible heat engine becomes:. Since this leg of the process va. → vb is irreversible, the entire process is irreversible. reestablish ing the initial state a requires more heat energy than the work done by the system in an irreversible process. in the initially rapid expansion, va → vc, the gas pressure will drop precipitously from pa to pc=pb.

Comments are closed.