Moderna Requests Fda Approval For Fourth Covid 19 Dose
Moderna Completes Submission For Full Fda Approval Of Covid 19 Vaccine Nation mar 18, 2022 10:46 am edt. drugmaker moderna asked the food and drug administration on thursday to authorize a fourth shot of its covid 19 vaccine as a booster dose for all adults. the. Washington — drugmaker moderna asked the food and drug administration on thursday to authorize a fourth shot of its covid 19 vaccine as a booster dose for all adults the request is broader.
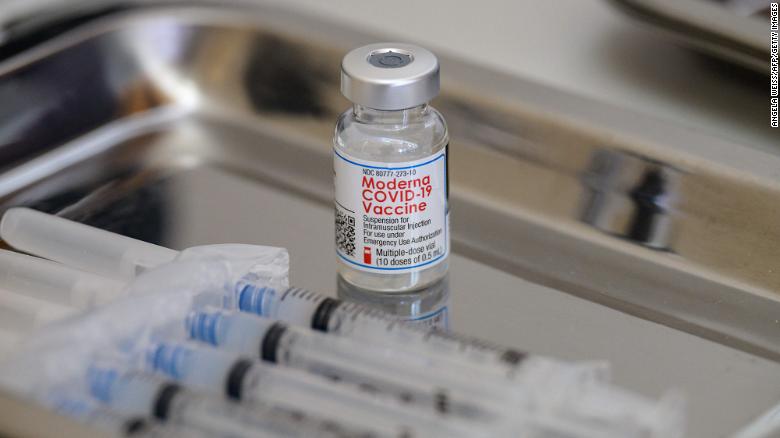
Moderna S Covid 19 Vaccine Receives Full Fda Approval Cnn Request is broader than pfizer's recent request for approval of booster for all seniors. a vial of the moderna covid 19 vaccine awaits administration at a vaccination clinic in los angeles on dec. Drugmaker moderna asked the food and drug administration on thursday to authorize a fourth shot of its covid 19 vaccine as a booster dose for all adults. the request is broader than rival pharmaceutical company pfizer’s request earlier this week for the regulator to approve a booster shot for all seniors. Cambridge, ma accesswire march 17, 2022 moderna, inc. (nasdaq:mrna), a biotechnology company pioneering messenger rna (mrna) therapeutics and vaccines, today announced that it has submitted a request to the u.s. food and drug administration (fda) for an amendment to the emergency use authorization (eua) to allow for a fourth dose of its covid 19 vaccine (mrna 1273) in adults 18 years of. Drugmaker moderna asked the food and drug administration on thursday to authorize a fourth shot of its covid 19 vaccine as a booster dose for all adults the request is broader than rival.

Comments are closed.