Moderna Seeks Approval For Second Covid 19 Booster Shot For Adults Npr
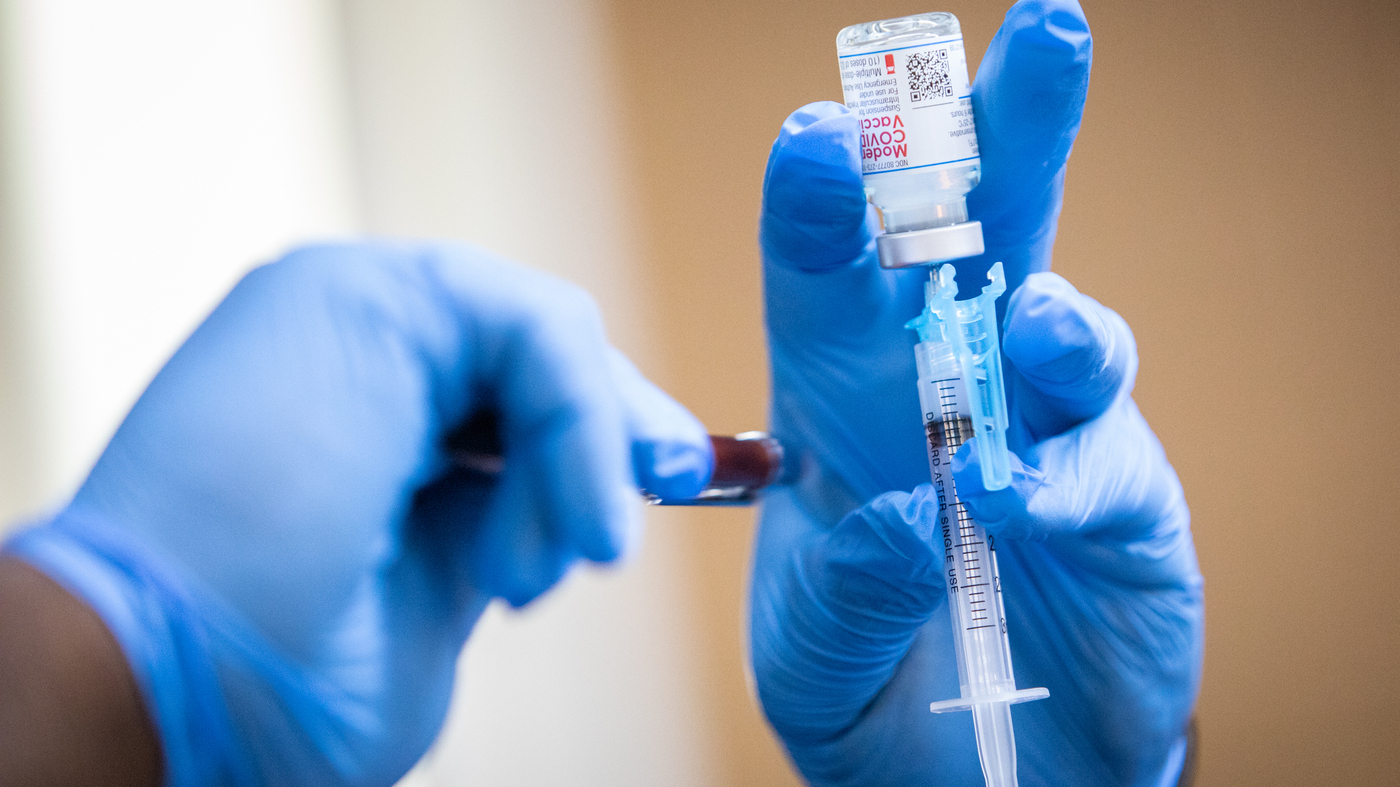
Moderna Seeks Approval For Second Covid 19 Booster Shot For Adults Npr Michael minasi kut. moderna is seeking approval for a fourth covid 19 vaccination shot, pending a grant for emergency authorization use from the food and drug administration, the company announced. If approved, this would be the second booster shot moderna has issued for people ages 18 and up. moderna seeks approval for a 2nd covid 19 booster shot for adults | hawai'i public radio search query show search.

Moderna Seeks Fda Authorization For A Second Booster Dose Of Its A moderna covid 19 vaccine is prepared during a pop up vaccine clinic at cristo rey church in east austin last summer. image: michael minasi kut moderna is seeking approval for. Moderna has asked the fda to authorize a booster of its covid 19 vaccine. a third shot of the moderna vaccine boosts protection across age groups, notably in older adults, the company says. a. Along with allowing a second shot for some adults, the cdc is also working to streamline its covid 19 vaccine recommendations: now most people are considered "up to date" if they've had one shot. Moderna is seeking an amendment of the fda’s emergency use authorization for its covid 19 vaccine to allow a fourth vaccine dose for any adults who’ve gotten an initial booster of any of the.
Moderna Requests Eua For A Second Covid 19 Booster Fox News Along with allowing a second shot for some adults, the cdc is also working to streamline its covid 19 vaccine recommendations: now most people are considered "up to date" if they've had one shot. Moderna is seeking an amendment of the fda’s emergency use authorization for its covid 19 vaccine to allow a fourth vaccine dose for any adults who’ve gotten an initial booster of any of the. Moderna announced thursday that it's asked the us food and drug administration for authorization for a second covid 19 booster shot for everyone 18 and older. Fda office of media affairs. 301 796 4540. consumer: 888 info fda. the fda approved a second covid 19 vaccine. the vaccine has been known as the moderna covid 19 vaccine; the approved vaccine will.
Moderna Completes Submission For Full Fda Approval Of Covid 19 Vaccine Moderna announced thursday that it's asked the us food and drug administration for authorization for a second covid 19 booster shot for everyone 18 and older. Fda office of media affairs. 301 796 4540. consumer: 888 info fda. the fda approved a second covid 19 vaccine. the vaccine has been known as the moderna covid 19 vaccine; the approved vaccine will.

Moderna Asks Fda For Authorization For A Second Booster For Adults

Comments are closed.