Oxidation Vs Reduction What Are Oxidation And Reduction Reactions In Everyday Life

Difference Between Oxidation And Reduction Redox Reaction Class 11 By mike crystal. an oxidation reduction reaction, or redox reaction, is a chemical reaction in which one or more electrons are transferred from one molecule or compound to another. the species that loses electrons is oxidized and usually a reducing agent; the species that gains electrons is reduced and is usually the oxidizing agent. everyday. In these reactions, one substance gets oxidized (loses electrons), while another simultaneously undergoes reduction (gains electrons). this dual occurrence isn’t mere coincidence; they’re interlinked events – when an atom donates an electron (oxidizes), another atom has to accept it (reduces). consider burning methane for instance: ch4.
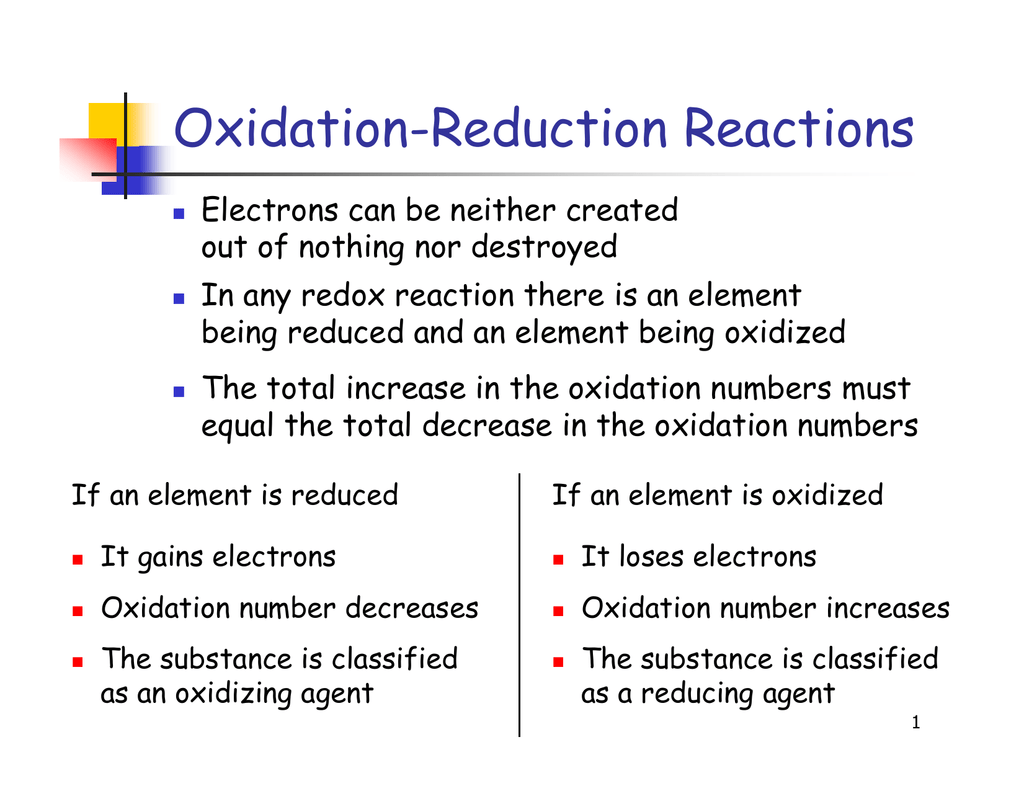
Difference Between Oxidation And Reduction The hydrogen ions are said to be reduced and the reaction is a reduction reaction. since both processes are going on at the same time, the initial reaction is called an oxidation reduction reaction. this type of reaction is also called a redox reaction (reduction oxidation). In fact, oxidation reduction reactions are intimately connected with the functioning of the natural environment. for example, photosynthesis, the conversion of light to chemical energy by plants, is a form of oxidation reduction reaction that produces two essentials of human life: oxygen and carbohydrates. An oxidation reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting. Oxidation reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. many such reactions are as common and familiar as fire, the rusting and dissolution of metals, the browning of fruit, and respiration and photosynthesis—basic life functions.

Oxidation Vs Reduction What Are Oxidation And Reduction Reactionsођ An oxidation reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. redox reactions are common and vital to some of the basic functions of life, including photosynthesis, respiration, combustion, and corrosion or rusting. Oxidation reduction reaction, any chemical reaction in which the oxidation number of a participating chemical species changes. many such reactions are as common and familiar as fire, the rusting and dissolution of metals, the browning of fruit, and respiration and photosynthesis—basic life functions. Corrosion is an example of the type of chemical reaction discussed in this chapter. although we usually think of corrosion as bad, the reaction it typifies can actually be put to good use. 14.2: oxidation reduction reactions oxidation reduction (redox) reactions involve the transfer of electrons from one atom to another. A simplified overall formula for photosynthesis is: 6co2 6h2o photons → c6h12o6 6o2 6 c o 2 6 h 2 o p h o t o n s → c 6 h 12 o 6 6 o 2. or simply. in this reaction, carbon dioxide is reduced to glucose, and water is oxidized to oxygen gas. other reactions convert the glucose to more complex carbohydrates, plant proteins, and oils.
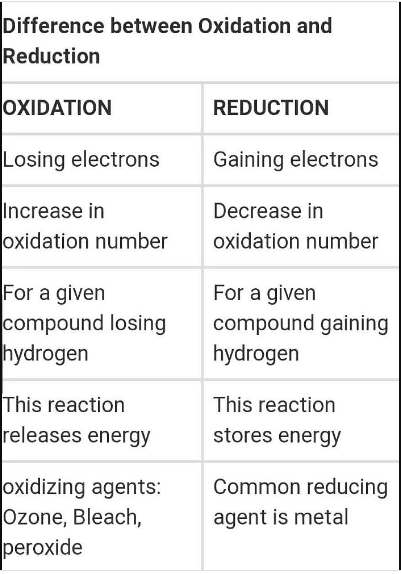
Difference Between Oxidation And Reduction Ox Science Corrosion is an example of the type of chemical reaction discussed in this chapter. although we usually think of corrosion as bad, the reaction it typifies can actually be put to good use. 14.2: oxidation reduction reactions oxidation reduction (redox) reactions involve the transfer of electrons from one atom to another. A simplified overall formula for photosynthesis is: 6co2 6h2o photons → c6h12o6 6o2 6 c o 2 6 h 2 o p h o t o n s → c 6 h 12 o 6 6 o 2. or simply. in this reaction, carbon dioxide is reduced to glucose, and water is oxidized to oxygen gas. other reactions convert the glucose to more complex carbohydrates, plant proteins, and oils.
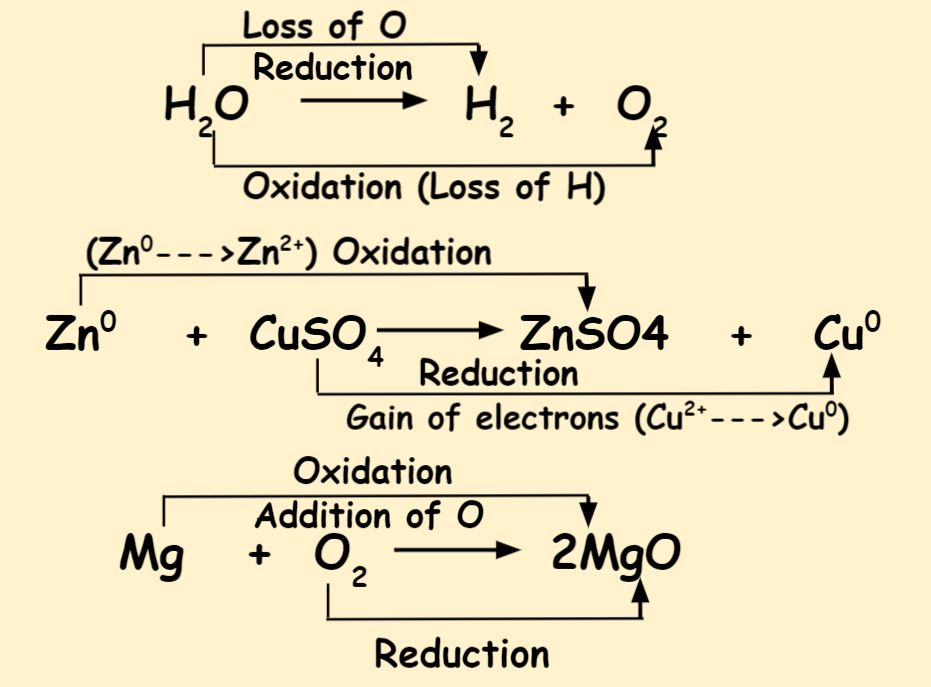
10 Differences Between Oxidation And Reduction Reaction Dewwool

Comments are closed.