Pdf T Cells Isolated From G Csf Treated Multiple Myeloma Patients Are

Pdf T Cells Isolated From G Csf Treated Multiple Myeloma Patients Are Patients. moreover, we found that car t cell fitness and anti tumor activity were unaffected when generated from g csf exposed t cells. overall, we showed that asct apher esis products are a suitable source of t cells for anti bcma car t cell manufacture. introduction multiple myeloma (mm) is a b cell malignancy that accounts for almost 1% of. Introduction. multiple myeloma (mm) is a b cell malignancy that accounts for almost 1% of all newly diagnosed cancers. 1 although treatment options for mm have undergone radical improvements over recent decades, the disease remains incurable, and relapse is inevitable in almost all cases. 2 chimeric antigen receptor (car) modified t cells that target b cell maturation antigen (bcma) have.
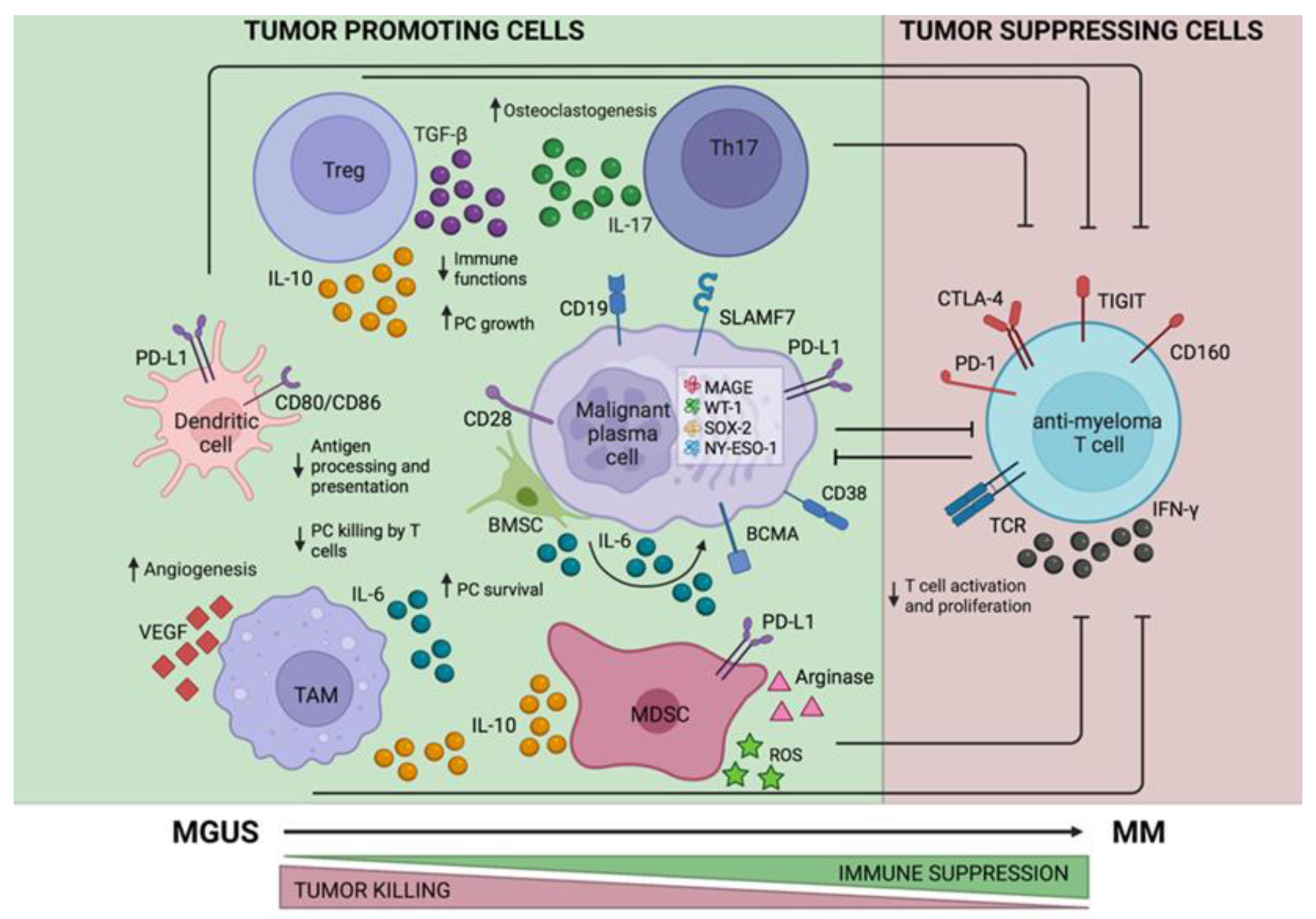
Ijms Free Full Text The Role Of T Cell Immunity In Monoclonal Autologous cell immunotherapy using b cell maturation antigen (bcma) targeted chimeric antigen receptor (car) t cells is an effective novel treatment for multiple myeloma (mm). this therapy has only been used for relapsed and refractory patients, at which stage the endogenous t cells used to produce the car t cells are affected by the. T cells isolated from g csf treated multiple myeloma patients are suitable for the generation of bcma directed car t cells anthony m. battram, aina oliver caldés, maria suárez lledó, miquel lozano, miquel bosch i crespo, núria martínez cibrián, joan cid, david f. moreno, luis gerardo. T cells were isolated from the blood of 9 patients with mm before and after 4 days of subcutaneous g csf administration (pre g csf and post g csf, respectively) prior to peripheral blood cd34 cell harvesting for an asct as consolidation after first line induction treatment. following stimulation with anti cd3 anti cd28 beads and il 2, t cells. Multiple myeloma (mm) is a plasma cell (pc) disease that leads to anemia, bone lesions, and eventually renal failure [].it is the second most common hematologic cancer, accounting for 10% of all.
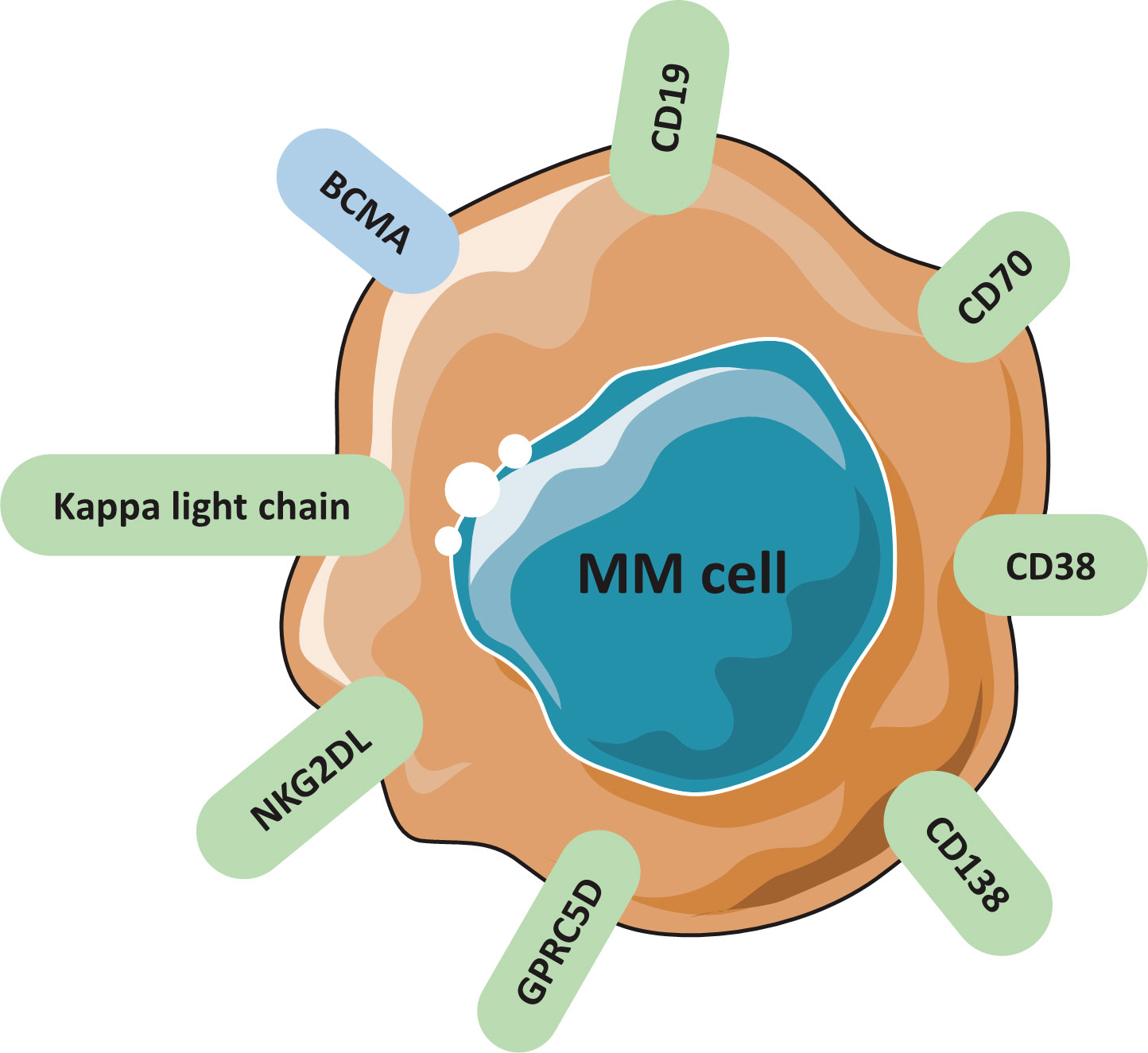
Frontiers Chimeric Antigen Receptor T Cell Therapy For Multiple Myeloma T cells were isolated from the blood of 9 patients with mm before and after 4 days of subcutaneous g csf administration (pre g csf and post g csf, respectively) prior to peripheral blood cd34 cell harvesting for an asct as consolidation after first line induction treatment. following stimulation with anti cd3 anti cd28 beads and il 2, t cells. Multiple myeloma (mm) is a plasma cell (pc) disease that leads to anemia, bone lesions, and eventually renal failure [].it is the second most common hematologic cancer, accounting for 10% of all. From 22 january 2018 to 30 october 2020, a total of 122 patients from 18 sites in five countries were enrolled and randomized 2:1 to receive either motixafortide g csf (80 patients) or placebo. This phase 3, multicenter, randomized (1:1), double blind, placebo controlled study evaluated the safety and efficacy of plerixafor with granulocyte colony stimulating factor (g csf) in mobilizing hematopoietic stem cells in patients with multiple myeloma. patients received g csf (10 microg kg) subcutaneously daily for up to 8 days.

The Safety And Clinical Effects Of Administering A Multiantigen From 22 january 2018 to 30 october 2020, a total of 122 patients from 18 sites in five countries were enrolled and randomized 2:1 to receive either motixafortide g csf (80 patients) or placebo. This phase 3, multicenter, randomized (1:1), double blind, placebo controlled study evaluated the safety and efficacy of plerixafor with granulocyte colony stimulating factor (g csf) in mobilizing hematopoietic stem cells in patients with multiple myeloma. patients received g csf (10 microg kg) subcutaneously daily for up to 8 days.

Multiple Myeloma With Gвђђcsf Production Mimicking Chronic Neutrophilic

Comments are closed.