Periodic Table Most Reactive Metals

Most Reactive Element On The Periodic Table 2024 Periodic Table The most reactive metal on the periodic table is francium. francium, however, is a laboratory produced element and only minute quantities have been made, so for all practical purposes, the most reactive metal is cesium. cesium reacts explosively with water, though it is predicted francium would react even more vigorously. Cesium is the most reactive metal, while fluorine is the most reactive nonmetal. the most reactive metal is cesium, while the most reactive nonmetal is fluorine. so, the most reactive element on the periodic table is either one of these elements. but, reactivity means different things to different chemists, plus it depends on a few factors.

The Most Reactive Metals Are Located In Which Area Of The Periodic Alkali metal are most reactive metals. down the reactivity increases. francium is most reactive element in periodic table. however, francium is artificial or only few quantities have produced right now, so after the francium, cesium is most reactive metal. The most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide: 2 na (s) 2 h 2 o (l) →2 naoh (aq) h 2 (g) metals in the middle of the reactivity series, such as iron , will react with acids such as sulfuric acid (but not water at normal temperatures) to give hydrogen and a metal salt , such as iron(ii) sulfate :. Most transition metals react with acids, but do not react with steam. the noble metals only react with powerful oxidizers, such as aqua regia. most reactive and least reactive metals. from the table, note that the most reactive metal on the periodic table is cesium. the least reactive metal is platinum. The alkali metals, found in group 1 of the periodic table (formally known as group ia), are so reactive that they are generally found in nature combined with other elements. the alkali metals are shiny, soft, highly reactive metals at standard temperature and pressure.
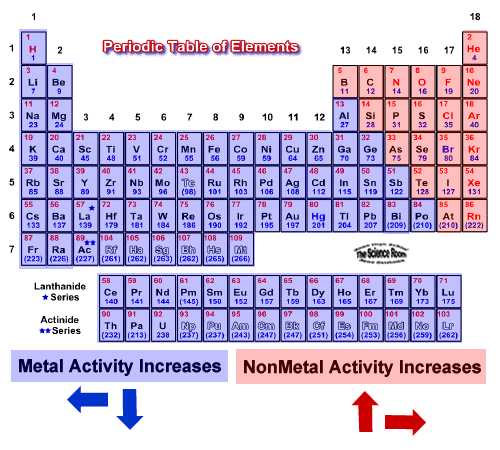
What Is The Most Reactive Metal On The Periodic Table Socratic Most transition metals react with acids, but do not react with steam. the noble metals only react with powerful oxidizers, such as aqua regia. most reactive and least reactive metals. from the table, note that the most reactive metal on the periodic table is cesium. the least reactive metal is platinum. The alkali metals, found in group 1 of the periodic table (formally known as group ia), are so reactive that they are generally found in nature combined with other elements. the alkali metals are shiny, soft, highly reactive metals at standard temperature and pressure. Fluorine is identified as the most electronegative element in the periodic table, making it the strongest oxidizing agent. it is the most reactive non metal. fluorine is so reactive that it can burn substances that one would generally think of as non flammable! it can burn glass, water and even sand! its promiscuity makes it impossible to store. The activity of metals. the primary difference between metals is the ease with which they undergo chemical reactions. the elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. lithium, sodium, and potassium all react with water, for example.
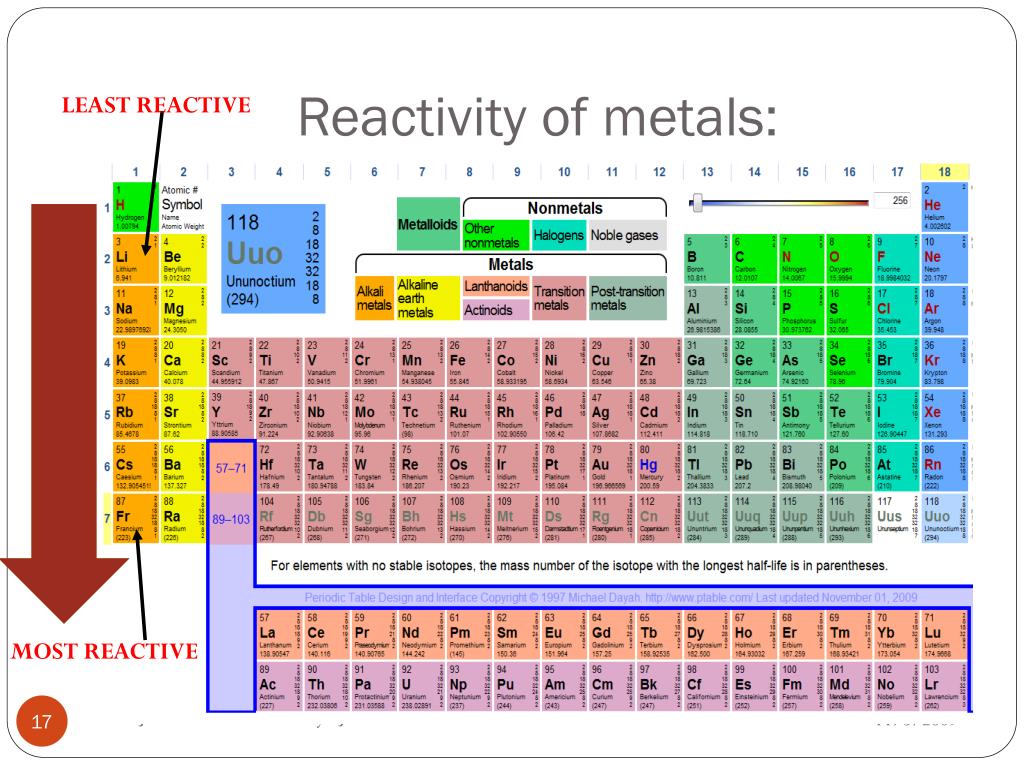
Periodic Table Most Reactive Metals Fluorine is identified as the most electronegative element in the periodic table, making it the strongest oxidizing agent. it is the most reactive non metal. fluorine is so reactive that it can burn substances that one would generally think of as non flammable! it can burn glass, water and even sand! its promiscuity makes it impossible to store. The activity of metals. the primary difference between metals is the ease with which they undergo chemical reactions. the elements toward the bottom left corner of the periodic table are the metals that are the most active in the sense of being the most reactive. lithium, sodium, and potassium all react with water, for example.
Periodic Table Reactivity Series Brokeasshome

Comments are closed.