Periodic Table Reactivity Chart Periodic Table Timeline

The Metal Reactivity Series Chemistry Classroom Reactivity is a measure of how easily an element will combine with other elements to form compounds. some elements are unreactive and need energy putting in others will react spontaneously and easily. the size of the nucleus determines the chemical reactivity of the element due to its ability to hold onto electrons and attract electrons. Figure 1.6.1 1.6. 1: the periodic table. red elements are the alkali and alkaline earth metals (s block). yellow elements are the transition metals (d block). orange elements are the lanthanides and actinides (f block). green, blue and purple elements are the p block; together with the s block, they are called the main group elements.
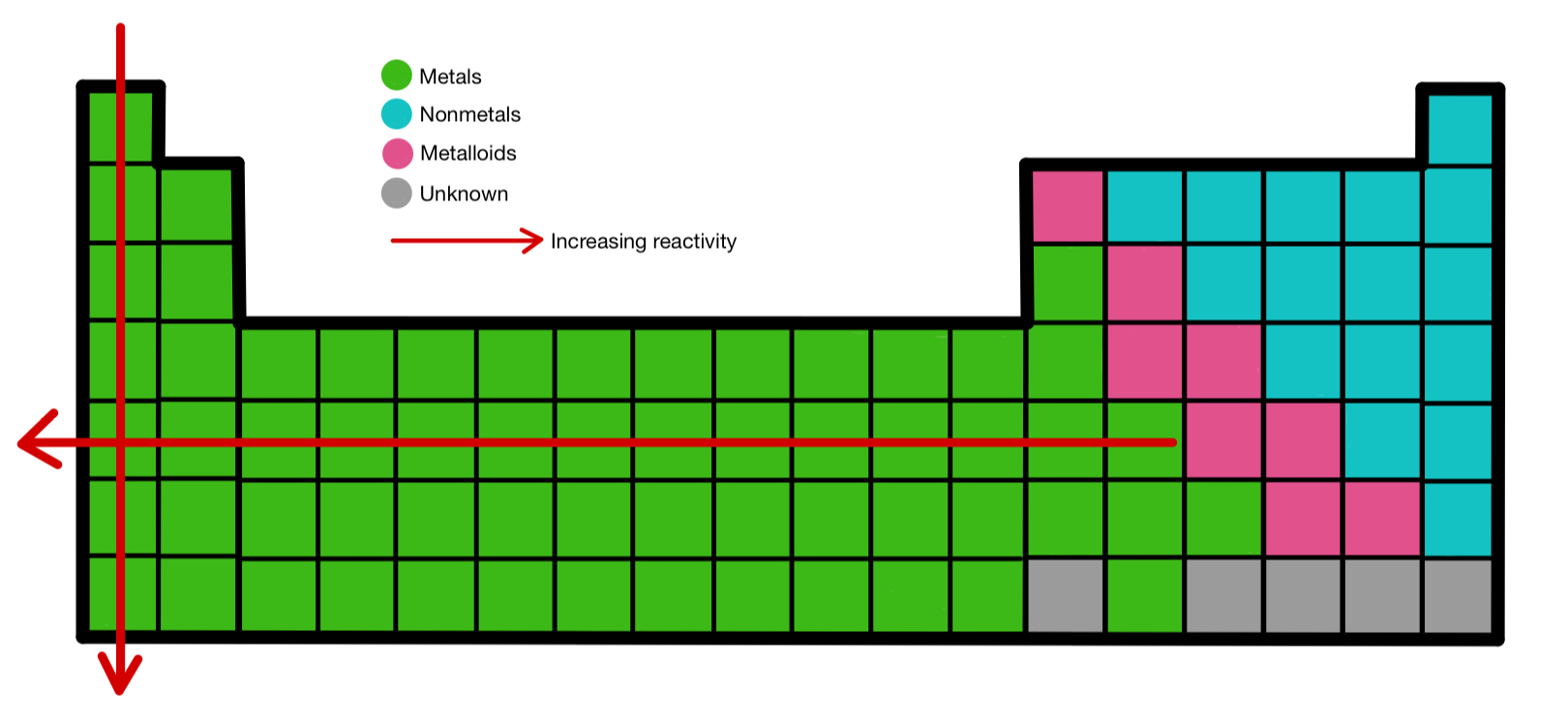
Predicting Reactivity Using The Periodic Table вђ Overview Expii In this article, you will learn about the reactivity series, including its significance and its applications. after reading this article, you will be able to understand the nature of the reactivity series as well as its uses and functions. topics covered in other articles. interactive periodic table; periodic trends made easy; atomic radius trend. The data provided by the reactivity series can be used to predict whether a metal can displace another in a single displacement reaction. it can also be used to obtain information on the reactivity of metals towards water and acids. 3,093. a chart of the reactivity series of common metals is provided below. metals tend to readily lose electrons. Atomic radius: atomic radius is a term describing the distance between an atom’s nucleus, and its outermost electron shell. several factors affect this distance; including the number of an element, and the number of electron shells. through periodic trends, the atomic radius increases in size further left of a period, and lower down a group. From the table, note that the most reactive metal on the periodic table is cesium. the least reactive metal is platinum. how to use the metal activity series – example problems. so, a metal that is higher on the activity series replaces one lower on the series. it does not replace a metal higher on the series.
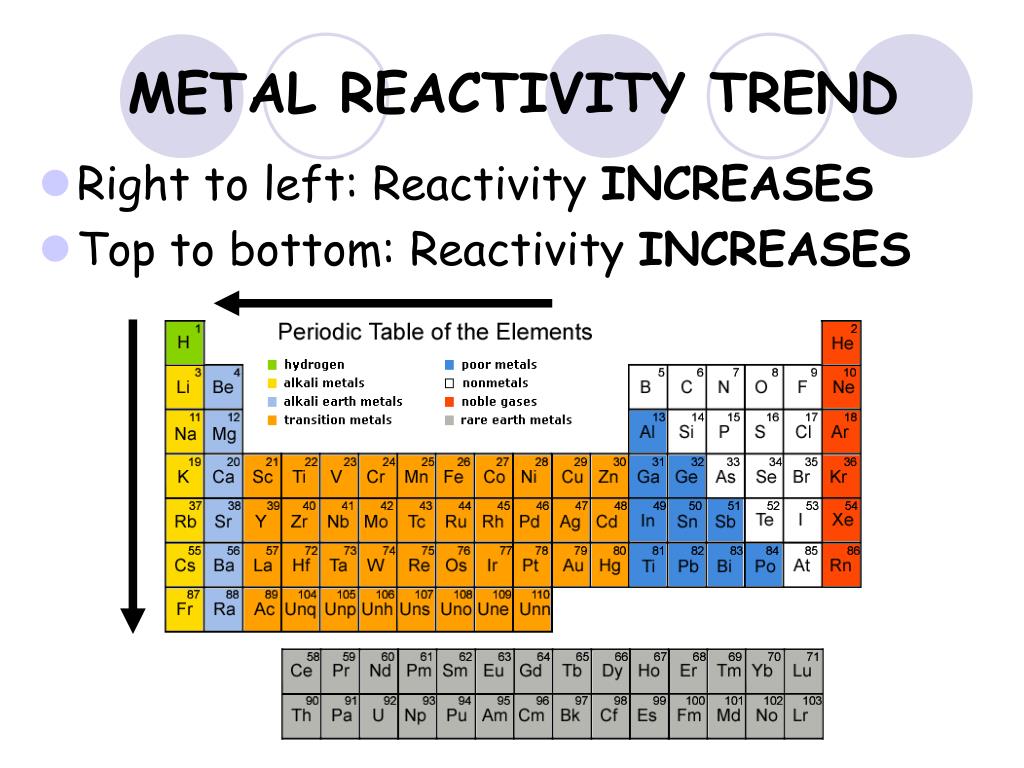
Periodic Table Trends Reactivity Periodic Table Timeline Atomic radius: atomic radius is a term describing the distance between an atom’s nucleus, and its outermost electron shell. several factors affect this distance; including the number of an element, and the number of electron shells. through periodic trends, the atomic radius increases in size further left of a period, and lower down a group. From the table, note that the most reactive metal on the periodic table is cesium. the least reactive metal is platinum. how to use the metal activity series – example problems. so, a metal that is higher on the activity series replaces one lower on the series. it does not replace a metal higher on the series. Periodic table. the royal society of chemistry's interactive periodic table features history, alchemy, podcasts, videos, and data trends across the periodic table. click the tabs at the top to explore each section. use the buttons above to change your view of the periodic table and view murray robertson’s stunning visual elements artwork. The activity series is a list of elements in decreasing order of their reactivity. since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. the table 6.11.1 below is an activity series of most common metals, and the table 6.11.2 is an activity series of the halogens.

Comments are closed.