Periodic Table Reactivity Series Of Metals Class 10 Periodic Table
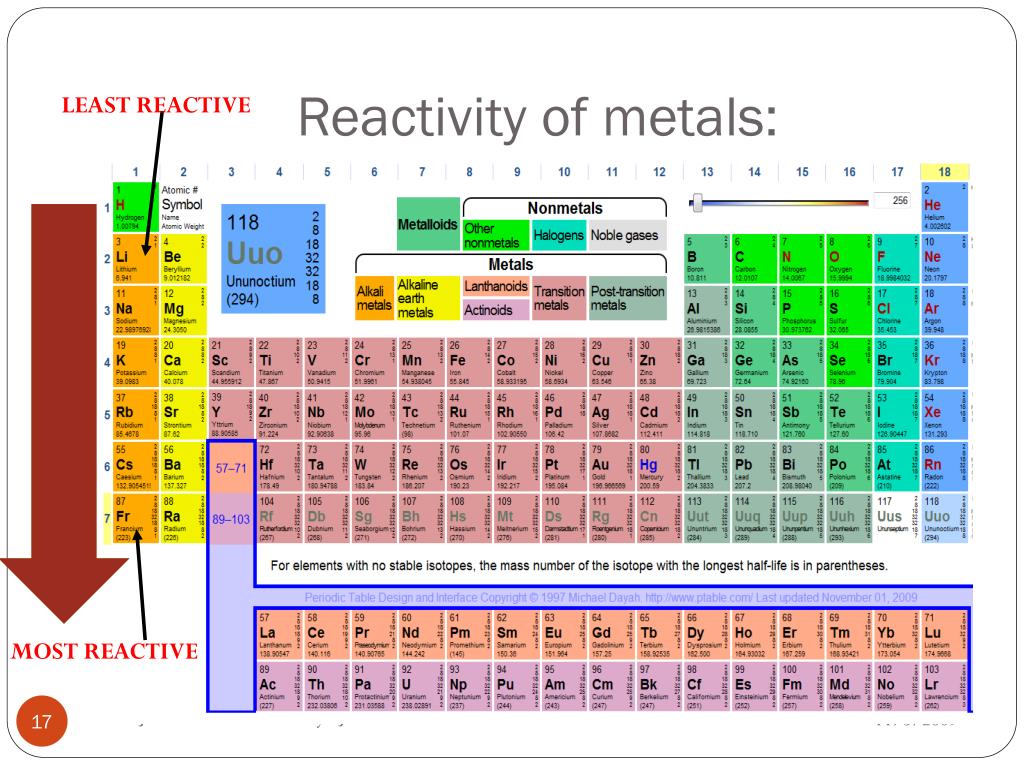
Series Periodic Table Chemistry Touchladeg The data provided by the reactivity series can be used to predict whether a metal can displace another in a single displacement reaction. it can also be used to obtain information on the reactivity of metals towards water and acids. 3,093. a chart of the reactivity series of common metals is provided below. metals tend to readily lose electrons. The activity series of metals or reactivity series is a list of metals from most reactive to least reactive. knowing the activity series helps you predict whether or not a chemical reaction occurs. specifically, use it for identifying whether a metal reacts with water or acid or whether it replaces another metal in a reaction.
Periodic Table Reactivity Chart Periodic Table Timeli Vrogue Co The reactivity series of metals is a chart listing metals in order of decreasing reactivity. in general, the more reactive a metal is: this table summarises the reactions of some metals in the. Reactivity is the tendency of chemical substances to form products by itself . reactivity series is an arrangement of metals and non metals based on their tendencies to react with certain tendencies. the elements are arranged in decreasing order of their reactivity . the arrangement of metals in a vertical column in the degree of their. So, by reactivity series, you can tell which metal will displace another metal. the reaction between metals and water – metals from potassium to calcium can react with cold water and release hydrogen gas. chemical equations for the reaction of k and ca with cold water are: reaction 1: k (s) h2o (l) → koh (aq) ½ h2 (g) potassium cold. The activity series is a list of elements in decreasing order of their reactivity. since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. the table \(\pageindex{1}\) below is an activity series of most common metals, and the table \(\pageindex{2}\) is an activity series of the.
Periodic Table Reactivity Of Elements Periodic Table Timeline So, by reactivity series, you can tell which metal will displace another metal. the reaction between metals and water – metals from potassium to calcium can react with cold water and release hydrogen gas. chemical equations for the reaction of k and ca with cold water are: reaction 1: k (s) h2o (l) → koh (aq) ½ h2 (g) potassium cold. The activity series is a list of elements in decreasing order of their reactivity. since metals replace other metals, while nonmetals replace other nonmetals, they each have a separate activity series. the table \(\pageindex{1}\) below is an activity series of most common metals, and the table \(\pageindex{2}\) is an activity series of the. Interactive periodic table of the chemical elements; properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more. reactivity series periodic table. Table of contents. the reactivity series of metals is a list of metals arranged in their order of reactivity from highest to lowest. at the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. at the bottom are the least reactive metals. during a single displacement reaction, a metal higher.
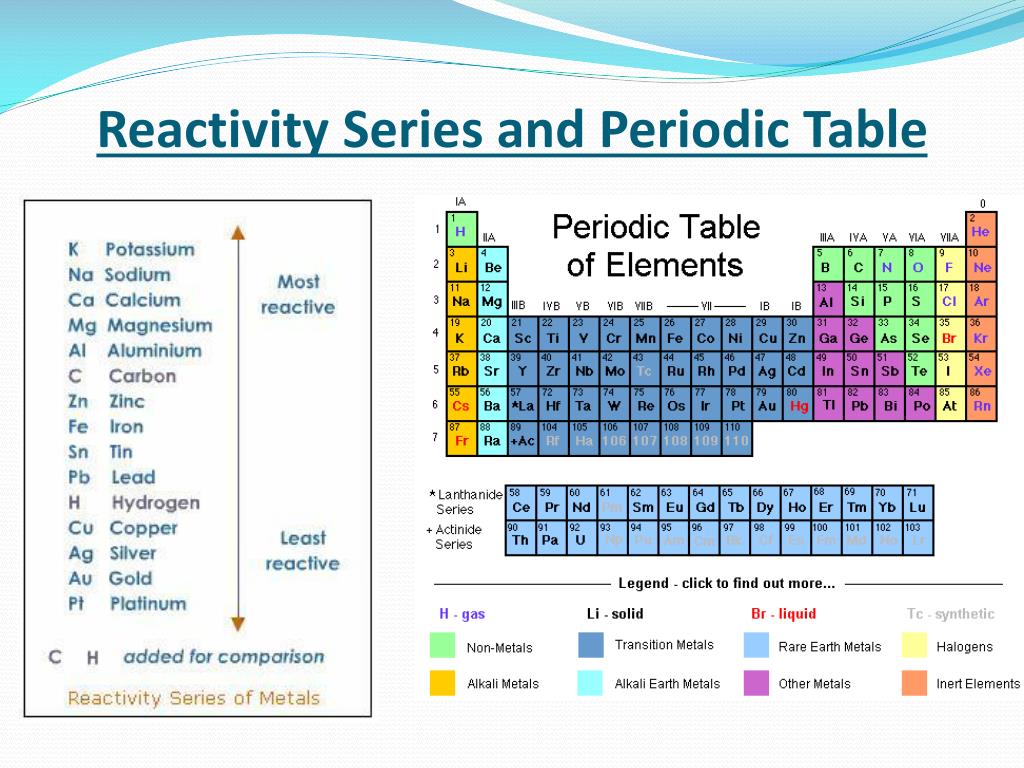
Series Periodic Table Chemistry Ladegjd Interactive periodic table of the chemical elements; properties, history, name origin, facts, applications, isotopes, electronic configuation, crystal structure, hazards and more. reactivity series periodic table. Table of contents. the reactivity series of metals is a list of metals arranged in their order of reactivity from highest to lowest. at the top of the list are the highly reactive metals that lose electrons during a chemical reaction to form ions. at the bottom are the least reactive metals. during a single displacement reaction, a metal higher.

Comments are closed.