Phases Of Clinical Trials Pharmaceutical Guidance
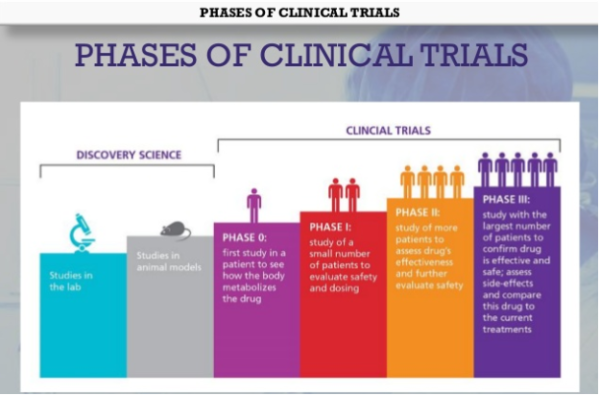
Phases Of Clinical Trials Pharmaceutical Guidance The drug development process. step 3: clinical research. while preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will. Clinical trials guidance documents. guidance documents listed below represent the agency's current thinking on the conduct of clinical trials, good clinical practice and human subject protection.
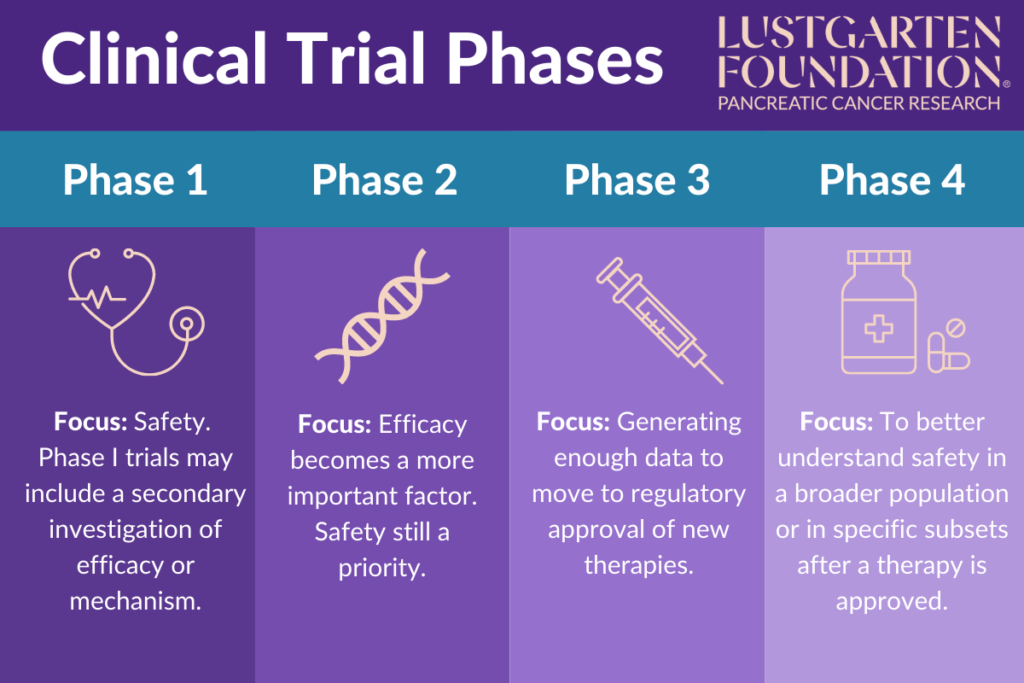
Clinical Trial Phases Step 1 discovery and development. discovery and development research for a new drug begins in the laboratory. more information. step 2 preclinical research. preclinical research drugs undergo. Key points of phase iii clinical trials. most phase iii clinical trials include a large number of patients, at least several hundred. these studies are often done in many places across the country (or even around the world) at the same time. phase iii clinical trials are more likely to be offered in local community hospitals and doctor's offices. The clinical trial process involves protocol development, designing a case record report form (crf), and functioning of institutional review boards (irbs). it also includes data management and the monitoring of clinical trial site activities. the crf is the most significant document in a clinical study. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and.
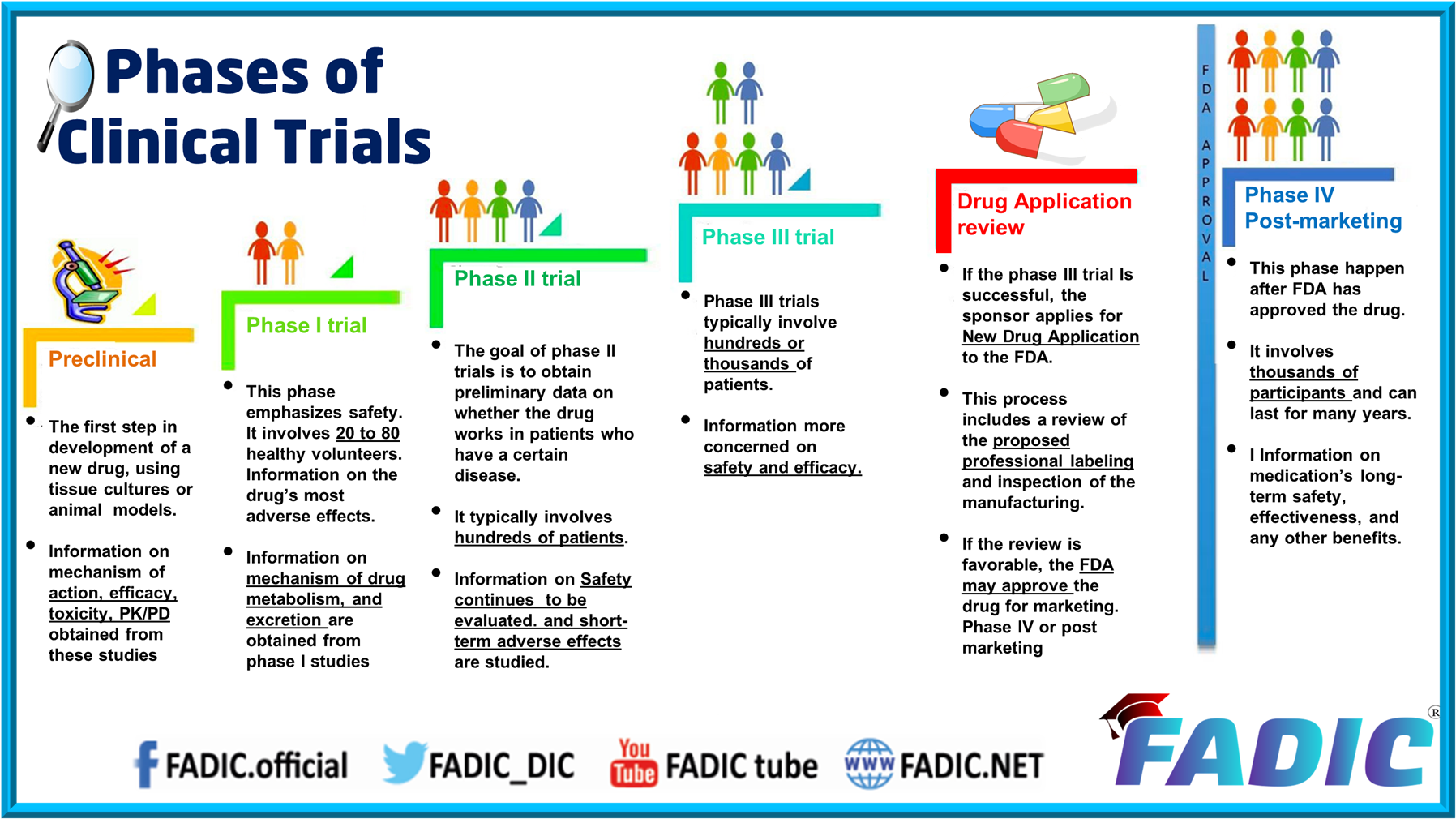
Phases Of Clinical Trials 30 Minutes E Course The clinical trial process involves protocol development, designing a case record report form (crf), and functioning of institutional review boards (irbs). it also includes data management and the monitoring of clinical trial site activities. the crf is the most significant document in a clinical study. Clinical trials follow a particular timeline, from early, small scale, phase 1 studies to late stage, large scale, phase 3 studies.1 while there are many steps involved in the development of new drugs, clinical trials, which make up clinical research, are the part of drug development that involves people. here we describe the key goals and. Pharmacology phase i trials into a single document.updated and edited in 2018 on behalf of and with the abpi experimental medicine expert network by: eric helmer, oliver. r, juliet mccolm and odile dewit.acknowledgements:we thank the many stakeholders from industry, regulators and professional organisations who provided feedback in re. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. the guidelines should be considered and used in an integrated, holistic way rather than focusing on only one guideline or subsection.
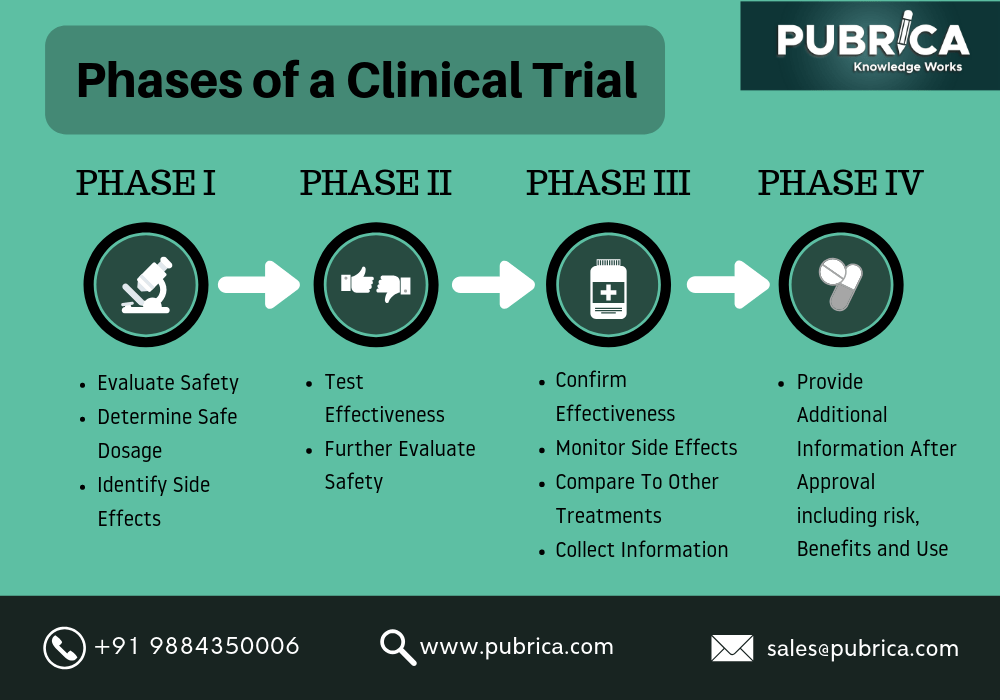
Phase 2 Clinical Trial вђ Academy Pharmacology phase i trials into a single document.updated and edited in 2018 on behalf of and with the abpi experimental medicine expert network by: eric helmer, oliver. r, juliet mccolm and odile dewit.acknowledgements:we thank the many stakeholders from industry, regulators and professional organisations who provided feedback in re. Ich e8 provides an overall introduction to clinical development, designing quality into clinical studies and focusing on those factors critical to the quality of the studies. the guidelines should be considered and used in an integrated, holistic way rather than focusing on only one guideline or subsection.

Comments are closed.