Ppt Water Hardness Determination With Edta Powerpoint Presentation

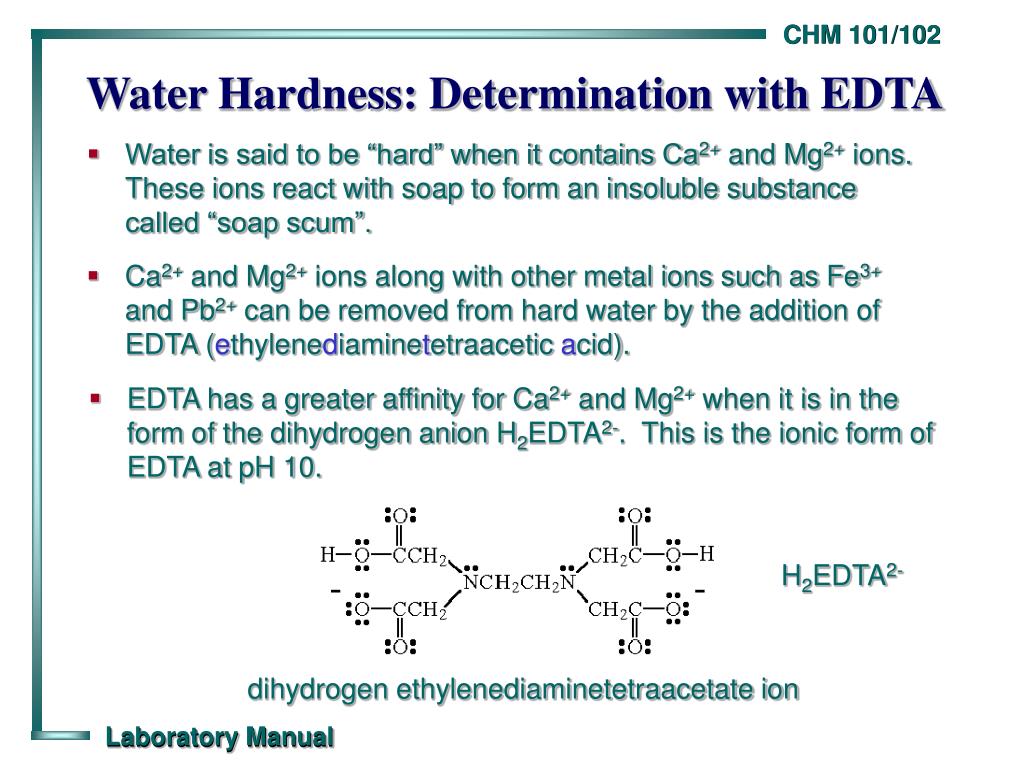
Ppt Water Hardness Determination With Edta Powerpoint Presentation Water hardness: determination with edta • using a volumetric pipet • squeeze the pipet bulb and place the silicone end over the top of the pipet. do not force the pipet into the bulb. • draw liquid up into the pipet until it is just above the calibration mark. slide the bulb off the pipet and place your index finger over the top of the pipet. 2. chm 101 102 water hardness: determination with edta water is said to be “hard” when it contains ca2 and mg2 ions. these ions react with soap to form an insoluble substance called “soap scum”. ca2 and mg2 ions along with other metal ions such as fe3 and pb2 can be removed from hard water by the addition of edta (ethylenediaminetetraacetic acid). edta has a greater affinity for.
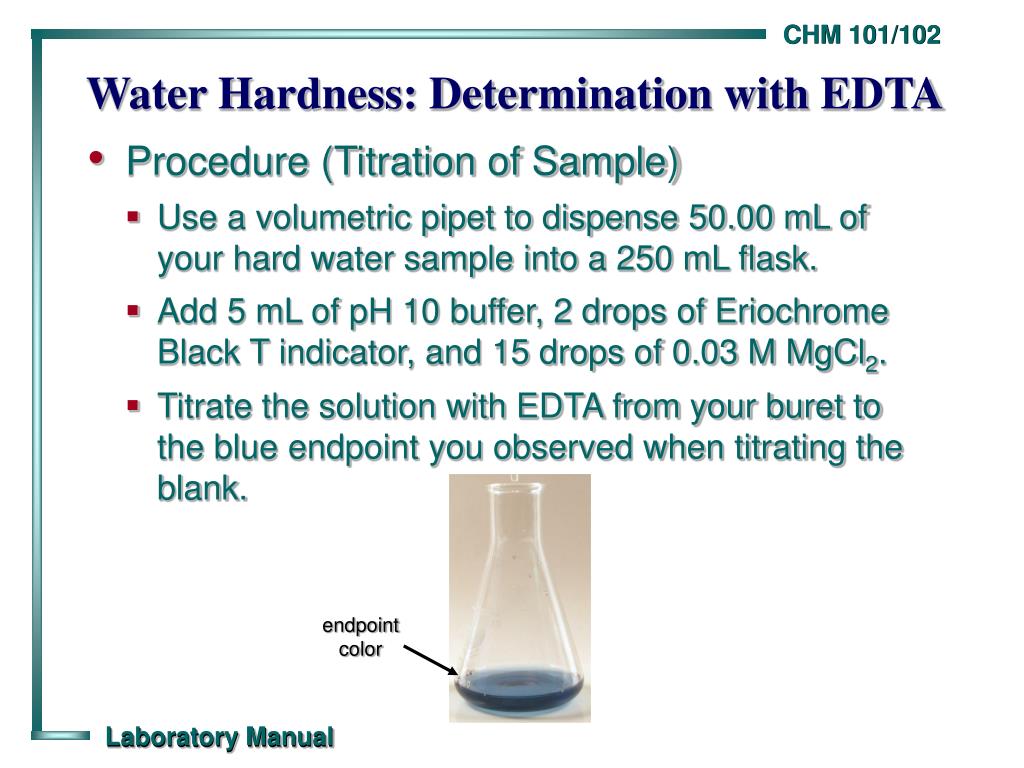
Ppt Water Hardness Determination With Edta Powerpoint Presentation Determination of hardness of water | ppt. 7. hardness in water can be estimated by complexometric titration using sodium salt of edta and ebt indicator at ph = 9 10. ebt forms an wine red colored weak unstable complex with ca2 mg2 ions, which upon titrating with edta, results in the breaking of ebt ca2 mg2 unstable bond and formation of stable strong edta ca2 mg2 bond. the endpoint changes from wine red (ebt ca2 mg2 ) to steel. Add this solution to 16.9 g nh4cl and 143 ml conc. nh4oh with mixing and dilute to 250 ml with distilled water. store in a plastic bottle. label, “buffer solution for hardness determination”. dissolve 3.723 g analytical reagent grade edta disodium salt dihydrate in distilled water and dilute to 1000 ml. The edta reagent can be used to measure the total quantity of dissolved ca2 and mg2 ions in a water sample. thus the total hardness of a water sample can be estimated by titration with a standard solution of edta. suitable conditions for the titration are achieved by the addition of a buffer solution of ph 10.
Ppt Water Hardness Determination With Edta Powerpoint Presentation Add this solution to 16.9 g nh4cl and 143 ml conc. nh4oh with mixing and dilute to 250 ml with distilled water. store in a plastic bottle. label, “buffer solution for hardness determination”. dissolve 3.723 g analytical reagent grade edta disodium salt dihydrate in distilled water and dilute to 1000 ml. The edta reagent can be used to measure the total quantity of dissolved ca2 and mg2 ions in a water sample. thus the total hardness of a water sample can be estimated by titration with a standard solution of edta. suitable conditions for the titration are achieved by the addition of a buffer solution of ph 10. Presentation transcript. hardness of water 1st step: the calcium ion coordinates with the indicator (eriochrome black t). h2in ca2 ↔ cain 2h1 2nd step: the edta chelates the calcium ion and releases the indicator. edta (aq) cain– (aq) 2h (aq) → h2in– (aq) caedta (aq) pink blue. sample calculations: 1st standardization of. Water hardness: determination with edta. water hardness: determination with edta. general chemistry 101 102 laboratory manual university of north carolina at wilmington. purpose. to determine the “hardness” of a water sample using an edta titration. 3.64k views • 13 slides.

Comments are closed.