Reaction Rates And Stoichiometry Chemistry Tutorial

Reaction Rates And Stoichiometry Chemistry Tutorial Youtube This chemistry tutorial includes examples of calculating average reaction rates as well as calculating reaction rates of reactants or products relative to ot. 18.1 chemical reaction rates. a rate is a measure of how some property varies with time. speed is a familiar rate that expresses the distance traveled by an object in a given amount of time. wage is a rate that represents the amount of money earned by a person working for a given amount of time. likewise, the rate of a chemical reaction is a.

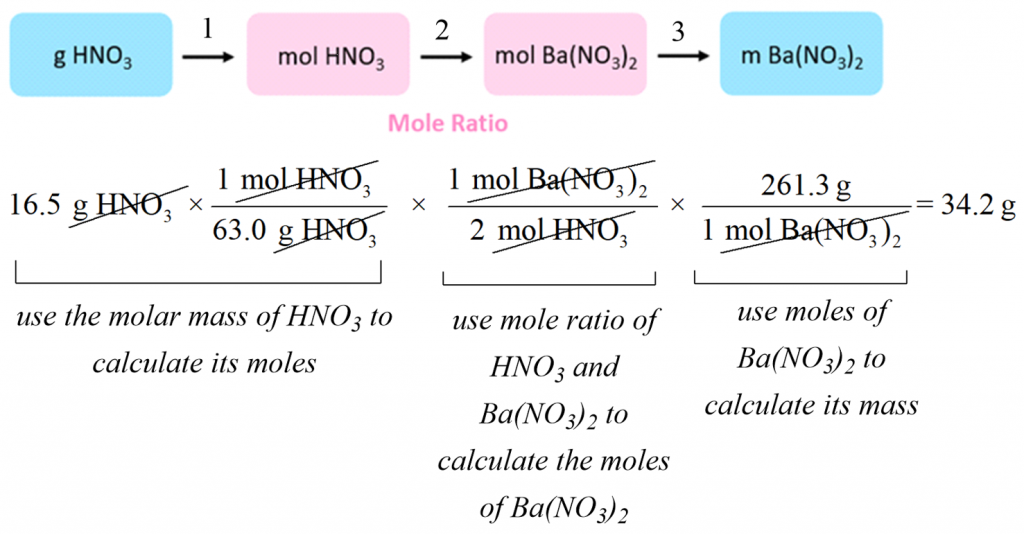
19 3 Reaction Rates And Stoichiometry Youtube Mgcl 2(aq) 2naoh(aq) → mg(oh) 2(s) 2nacl(aq) solution. the approach used previously in examples 7.3.1 and 7.3.2 is likewise used here; that is, we must derive an appropriate stoichiometric factor from the balanced chemical equation and use it to relate the amounts of the two substances of interest. in this case, however, masses (not molar. Reaction stoichiometry allows us to determine the amount of substance that is consumed or produced by a reaction. the following video considers the first part of this: how much of a reactant is consumed in a chemical reaction. product formation is discussed elsewhere. reaction stochiometry movie text. the chemcollective site and its contents. Problem 5.2.1.1 5.2.1. 1. write the balanced equation and determine the information requested. don't worry about state symbols in these reactions. the number of moles and the mass (in grams) of chlorine, cl 2, required to react with 10.0 g of sodium metal, na, to produce sodium chloride, nacl. the number of moles and the mass (in milligrams) of. 00:00 average, instantaneous, and initial rates02:14 graphical representations of rates03:22 production (consumption) rates and stoichiometry.

Rates Tutorial Reaction Rate Stoichiometry Problem 5.2.1.1 5.2.1. 1. write the balanced equation and determine the information requested. don't worry about state symbols in these reactions. the number of moles and the mass (in grams) of chlorine, cl 2, required to react with 10.0 g of sodium metal, na, to produce sodium chloride, nacl. the number of moles and the mass (in milligrams) of. 00:00 average, instantaneous, and initial rates02:14 graphical representations of rates03:22 production (consumption) rates and stoichiometry. Expressing a catalytic rate law in terms of conversion. calculating the equilibrium conversion for gas phase reaction. consider the following elementary reaction with k c and = 20 dm 3 mol and c a0 = 0.2 mol dm 3. pure a fed. calculate the equilibrium conversion, x e, for both a batch reactor and a flow reactor. 2nh3(g)→ n2(g) 3h2(g) 2nh 3 (g) → n 2 (g) 3h 2 (g) the relation between the reaction rates expressed in terms of nitrogen production and ammonia consumption, for example, is: − Δ mol nh3 Δt × 1 mol n2 2 mol nh3 = Δmol n2 Δt − Δ mol nh 3 Δ t × 1 mol n 2 2 mol nh 3 = Δ mol n 2 Δ t.

Reaction Rates And Stoichiometry Youtube Expressing a catalytic rate law in terms of conversion. calculating the equilibrium conversion for gas phase reaction. consider the following elementary reaction with k c and = 20 dm 3 mol and c a0 = 0.2 mol dm 3. pure a fed. calculate the equilibrium conversion, x e, for both a batch reactor and a flow reactor. 2nh3(g)→ n2(g) 3h2(g) 2nh 3 (g) → n 2 (g) 3h 2 (g) the relation between the reaction rates expressed in terms of nitrogen production and ammonia consumption, for example, is: − Δ mol nh3 Δt × 1 mol n2 2 mol nh3 = Δmol n2 Δt − Δ mol nh 3 Δ t × 1 mol n 2 2 mol nh 3 = Δ mol n 2 Δ t.
Stoichiometry Of Chemical Reactions Chemistry Steps

Comments are closed.