Solved A Catalyst Will Be Consumed In The Chemical Reaction Decrease

Solved A Catalyst Will Be Consumed In The Chemical Reaction Decrease A catalyst will be consumed in the chemical reaction o decrease the reaction's rate lower the activation energy of the chemical reaction o not change the rate at which equilibrium is established. your solution’s ready to go!. The reactant in an enzyme catalyzed reaction is called a substrate. enzyme inhibitors cause a decrease in the reaction rate of an enzyme catalyzed reaction. 14.7: catalysis. catalysts participate in a chemical reaction and increase its rate. they do not appear in the reaction’s net equation and are not consumed during the reaction.
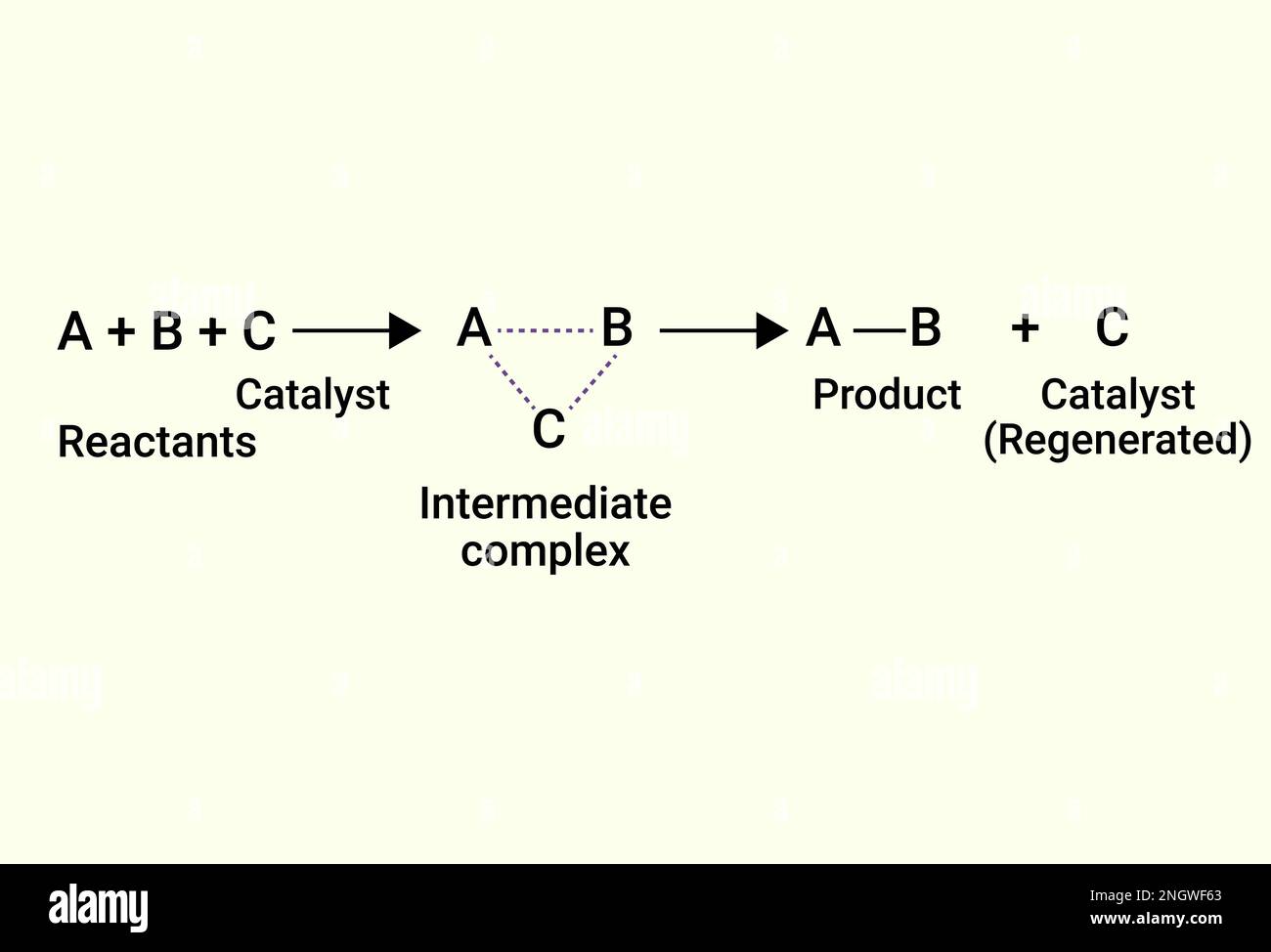
Chemical Reactions Of Catalyst And Product Stock Vector Image Art Alamy By saying catalysts are not consumed by reactions. is meant there is no stoichimetric ratio to reactants, consuming catalysts and forming from them catalytically inactive compound. by other words, some of reaction steps regenerates the original form of a catalyst, consumed by a prior step, so the net consumption is negligible. the transition. Other examples of catalytic reactions. the halogenation of benzene. benzene reacts with chlorine or bromine in the presence of a catalyst. the catalyst used can either be aluminum chloride or iron. strictly talking about iron is not a catalyst, because it gets permanently changed during the reaction. In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysis is the process of speeding up a reaction using a catalyst. the word “catalyst” comes from the greek word kataluein, which means to loosen or untie. british chemistry elizabeth fulhame first described the. Instead, the catalytic action occurs by adsorption, where catalyst acts as adsorbent and reactant acts as adsorbate. the whole process occurs in four steps: 1. during the process, the reactant molecules get attached or adsorbed on the catalyst’s surface by chemical bonding. 2. the adsorbed reactants get activated.
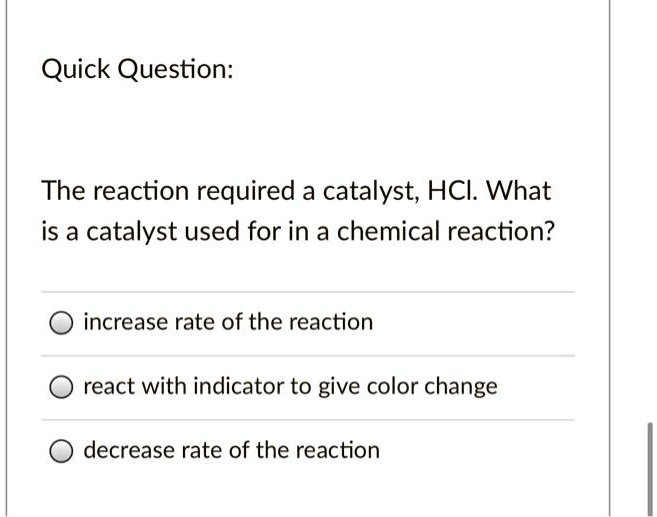
Solved Quick Question The Reaction Required A Catalyst Hci What Is A In chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. catalysis is the process of speeding up a reaction using a catalyst. the word “catalyst” comes from the greek word kataluein, which means to loosen or untie. british chemistry elizabeth fulhame first described the. Instead, the catalytic action occurs by adsorption, where catalyst acts as adsorbent and reactant acts as adsorbate. the whole process occurs in four steps: 1. during the process, the reactant molecules get attached or adsorbed on the catalyst’s surface by chemical bonding. 2. the adsorbed reactants get activated. The effect of a catalyst on rate of reaction. Learn how catalysts can lower the activation energy of a chemical reaction and increase the reaction rate, without being consumed or changed in the process. explore the concepts of reaction mechanisms, intermediates, and rate determining steps with examples and diagrams.

What Is A Catalyst Understand Catalysis The effect of a catalyst on rate of reaction. Learn how catalysts can lower the activation energy of a chemical reaction and increase the reaction rate, without being consumed or changed in the process. explore the concepts of reaction mechanisms, intermediates, and rate determining steps with examples and diagrams.
Solved The Function Of Catalyst In Chemical Reaction Is To A Increase

Comments are closed.