Thermodynamic Processes Isobaric Isochoric Isothermal And Adiabatic
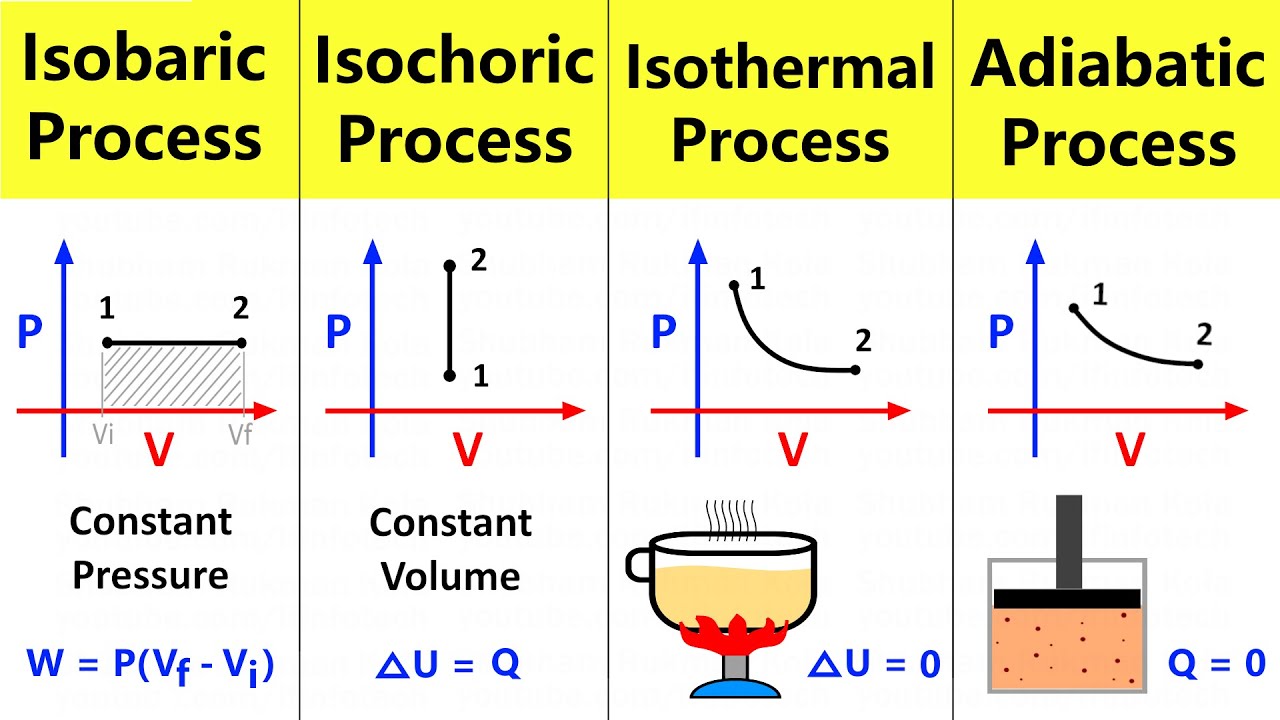
Thermodynamic Processes Isobaric Isochoric Isothermal And Adiabatic Although, the particles are moving faster the volume is also larger, making it possible to have the same pressure as in its initial state. when the pressure of a system remains constant during a thermodynamic process, the process is called isobaric. and illustration of such process is shown below. figure 4.4.4: example of an isobaric process. For adiabatic process, pv = constant i.e. p 1 v 1 γ = p 2 v 2 γ; the curve of the adiabatic process is steeper than that of the isothermal process. cyclic process. when a thermodynamic process is carried out in such a manner that after a series of state of changes the system is brought to its original state, then the process is known as a.

Thermodynamic Processes Definition Types And Solved Examples An isobaric process is a process where the pressure of the system does not change, whereas an isochoric process is a process where the volume of the system does not change. adiabatic processes in an adiabatic process , the system is insulated from its environment so that although the state of the system changes, no heat is allowed to enter or. Summary. when examining thermodynamic processes some simplifying assumptions may be applied to help describe and analyse a given system. these simplifications can be viewed as ‘ideal’ thermodynamic processes and include adiabatic, isenthalpic, isentropic, isobaric, isochoric, isothermal, isentropic, polytropic and reversible processes. Thermodynamic processes. we have four processes: isobaric, isochoric, isothermal, and adiabatic. isobaric process. an isobaric process is a process that occurs when pressure is constant. since the pressure is constant irrespective of the changes in volume, then the curve is a horizontal flat line, and the work is the rectangular area beneath it. Explain the differences among the simple thermodynamic processes—isobaric, isochoric, isothermal, and adiabatic. calculate total work done in a cyclical thermodynamic process. figure 15.6 beginning with the industrial revolution, humans have harnessed power through the use of the first law of thermodynamics, before we even understood it.

Comments are closed.