Total Water Hardness Using Edta Titration Youtube
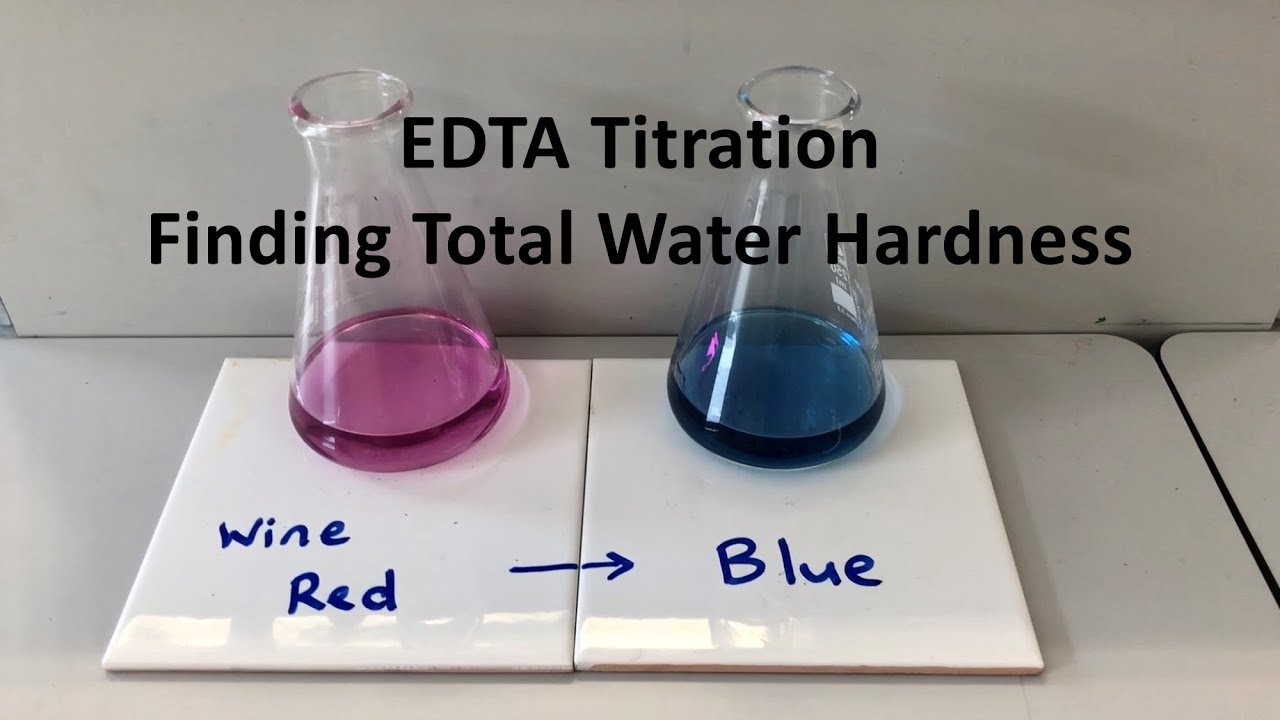
Total Water Hardness Using Edta Titration Youtube This is the classic method to determine the total water hardness over a titration with edta solution.patreon: patreon randomexperimentsintern. About press copyright contact us creators advertise developers terms privacy policy & safety how works test new features nfl sunday ticket press copyright.

Edta Titrimetric Method Experiment Measurement Of Total Hardness Ca By this animation you will learn how to find out total hardness in given water sample by titration method in easiest and short way.#hardnessofwater#findoutha. Therefore, mols of edta equals the total mols of ca 2 and mg 2 ions. to get the edta volume consumed by ions in the sample we should subtract the endpoint value of the blank titration from the endpoint value of the sample titration. the hardness of water is expressed as the milligrams of caco 3 in 1 liter of sample. Mass edta required = moles x molar mass = 2.500 x 10 3 x 372.244 = 0.9306 g. carefully transfer the edta to the volumetric flask. place a clean, dry, glass funnel in the neck of the volumetric flask. use a clean, dry, spatula to transfer some of the edta from the watchglass to the funnel. use a small amount of water from a wash bottle filled. Complexometric titration is one of the best ways of measuring total water hardness. at ph around 10 edta easily reacts with both calcium and magnesium in the same molar ratio (1:1). stability constant of calcium complex is a little bit higher, so calcium reacts first, magnesium later. thus, for the end point, we should use the same indicator we.

Estimation Of Hardness Of Water Ii Edta Complexometric Titration Ii Mass edta required = moles x molar mass = 2.500 x 10 3 x 372.244 = 0.9306 g. carefully transfer the edta to the volumetric flask. place a clean, dry, glass funnel in the neck of the volumetric flask. use a clean, dry, spatula to transfer some of the edta from the watchglass to the funnel. use a small amount of water from a wash bottle filled. Complexometric titration is one of the best ways of measuring total water hardness. at ph around 10 edta easily reacts with both calcium and magnesium in the same molar ratio (1:1). stability constant of calcium complex is a little bit higher, so calcium reacts first, magnesium later. thus, for the end point, we should use the same indicator we. Reaction: min – h 2 y 2 → hin 2 my 2 h . determination of hardness of water by edta method is father classified into four methods. ammonia buffer procedure. borate buffer procedure. low hardness procedure. calcium hardness procedure. ammonia buffer procedure: this type determination of hardness of water by edta method is. Water hardness is dominated by the presence of calcium and magnesium cations because of extensive contact that water has with soil and rock formations containing of these cations. because of this, total hardness is typically defined as the sum of calcium and magnesium cation concentrations and is expressed in mg l as calcium carbonate (caco3).

Edta Titration Complexomatric Titration Hardness Of Water Reaction: min – h 2 y 2 → hin 2 my 2 h . determination of hardness of water by edta method is father classified into four methods. ammonia buffer procedure. borate buffer procedure. low hardness procedure. calcium hardness procedure. ammonia buffer procedure: this type determination of hardness of water by edta method is. Water hardness is dominated by the presence of calcium and magnesium cations because of extensive contact that water has with soil and rock formations containing of these cations. because of this, total hardness is typically defined as the sum of calcium and magnesium cation concentrations and is expressed in mg l as calcium carbonate (caco3).

Total Water Hardness Using Edta Titration вђ Otosection

Comments are closed.