Vsepr Theory Chart

Vsepr Theory Table Learn how to use vsepr theory to organize molecules based on their geometric structures. see examples of linear, trigonal, tetrahedral, trigonal bipyramidal, octahedral, bent, trigonal pyramidal, seesaw, t shaped, square pyramidal, and square planar molecules. Learn how to use the vsepr model to predict the shapes of molecules and polyatomic ions with nonmetal or metal central atoms. see examples, diagrams, and a chart of common molecular geometries and ideal bond angles.
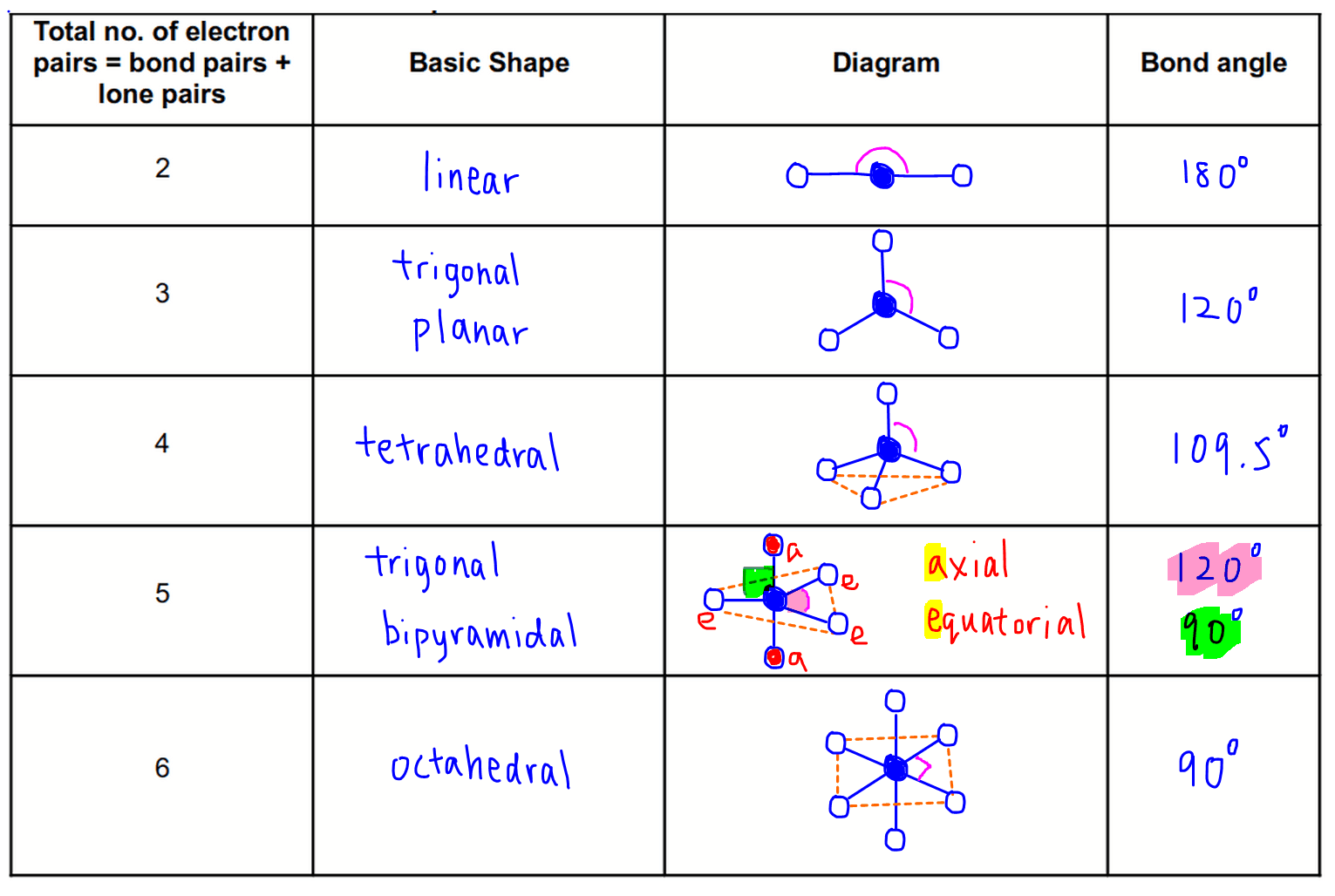
Vsepr Theory Chart Learn how to use the vsepr theory to predict the molecular shape and bond angles of polyatomic molecules. see the axe notation, the basic molecular structures, and the examples of vsepr theory. Learn how to use the vsepr model to predict molecular geometry based on the number of electron pairs around a central atom. download a free pdf chart with vsepr shapes and bond angles for different coordination numbers and numbers of atoms. Vsepr theory is a model that predicts the arrangement of electron pairs around central atoms in molecules based on their number and repulsion. it uses the steric number, the degree of repulsion, and the lone pair effect to determine the optimal geometry, such as linear, trigonal, tetrahedral, or trigonal bipyramidal. Learn how to use vsepr theory to predict the 3 d shape of molecules and ions based on the number and type of electron pairs around the central atom. see the vsepr notation, the electron pair geometry, and the molecular geometry tables with examples and diagrams.

Shape Of Molecules Vsepr Theory Affect Shape Of The Molecule Vsepr theory is a model that predicts the arrangement of electron pairs around central atoms in molecules based on their number and repulsion. it uses the steric number, the degree of repulsion, and the lone pair effect to determine the optimal geometry, such as linear, trigonal, tetrahedral, or trigonal bipyramidal. Learn how to use vsepr theory to predict the 3 d shape of molecules and ions based on the number and type of electron pairs around the central atom. see the vsepr notation, the electron pair geometry, and the molecular geometry tables with examples and diagrams. Learn how to predict the shape of simple molecules and polyatomic ions using valence shell electron pair repulsion (vsepr) theory. see examples, diagrams, and a table of common molecular shapes based on electron group geometry and number of electron groups. Learn how to predict the molecular structure and bond angles of small molecules using vsepr theory, which minimizes the repulsion between electron pairs. see examples, exercises, and a table of electron pair geometries and molecular structures.

Vsepr Theory Chart Learn how to predict the shape of simple molecules and polyatomic ions using valence shell electron pair repulsion (vsepr) theory. see examples, diagrams, and a table of common molecular shapes based on electron group geometry and number of electron groups. Learn how to predict the molecular structure and bond angles of small molecules using vsepr theory, which minimizes the repulsion between electron pairs. see examples, exercises, and a table of electron pair geometries and molecular structures.

Vsepr Theory Chart

Comments are closed.