What Is The Process Where Water Moves Across A Membrane From An Area Of

Cell Membrane Transport Osmosis Diffusion Britannica Figure \(\pageindex{1}\): osmosis: in osmosis, water always moves from an area of higher water concentration to one of lower concentration. in the diagram shown, the solute cannot pass through the selectively permeable membrane, but the water can. returning to the beaker example, recall that it has a mixture of solutes on either side of the. The process of osmosis over a semipermeable membrane.the blue dots represent particles driving the osmotic gradient. osmosis ( ɒ z ˈ m oʊ s ɪ s , us also ɒ s ) [1] is the spontaneous net movement or diffusion of solvent molecules through a selectively permeable membrane from a region of high water potential (region of lower solute concentration) to a region of low water potential.
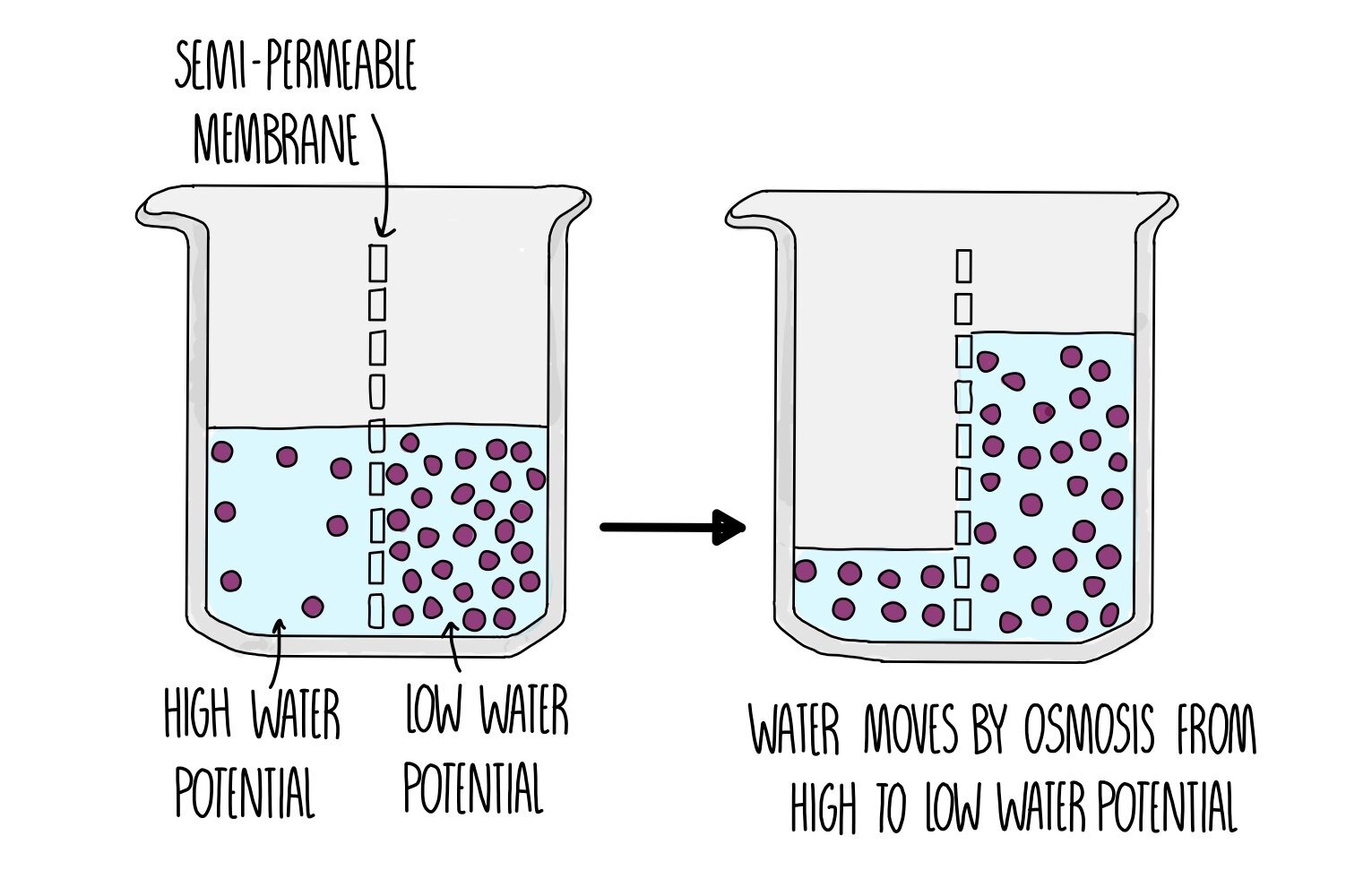
Movement Across Cell Membranes вђ The Science Hive Whereas diffusion transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the diffusion of solutes in the water. osmosis is a special case of diffusion. water, like other substances, moves from an area of higher concentration to one of lower concentration. Whereas diffusion transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the diffusion of solutes in the water. osmosis is a special case of diffusion. water, like other substances, moves from an area of higher concentration to one of lower concentration. Osmosis is the movement of free water molecules through a semipermeable membrane according to the water's concentration gradient across the membrane, which is inversely proportional to the solutes' concentration. in diffusion, the solutes move. in osmosis, the water moves. in both, the goal is the same: to balance out the solute concentration. Figure 6.5.1 6.5. 1: in osmosis, water always moves from an area of higher concentration (of water) to one of lower concentration (of water). in this system, the solute cannot pass through the selectively permeable membrane. a principle of diffusion is that the molecules move around and will spread evenly throughout the medium if they can.
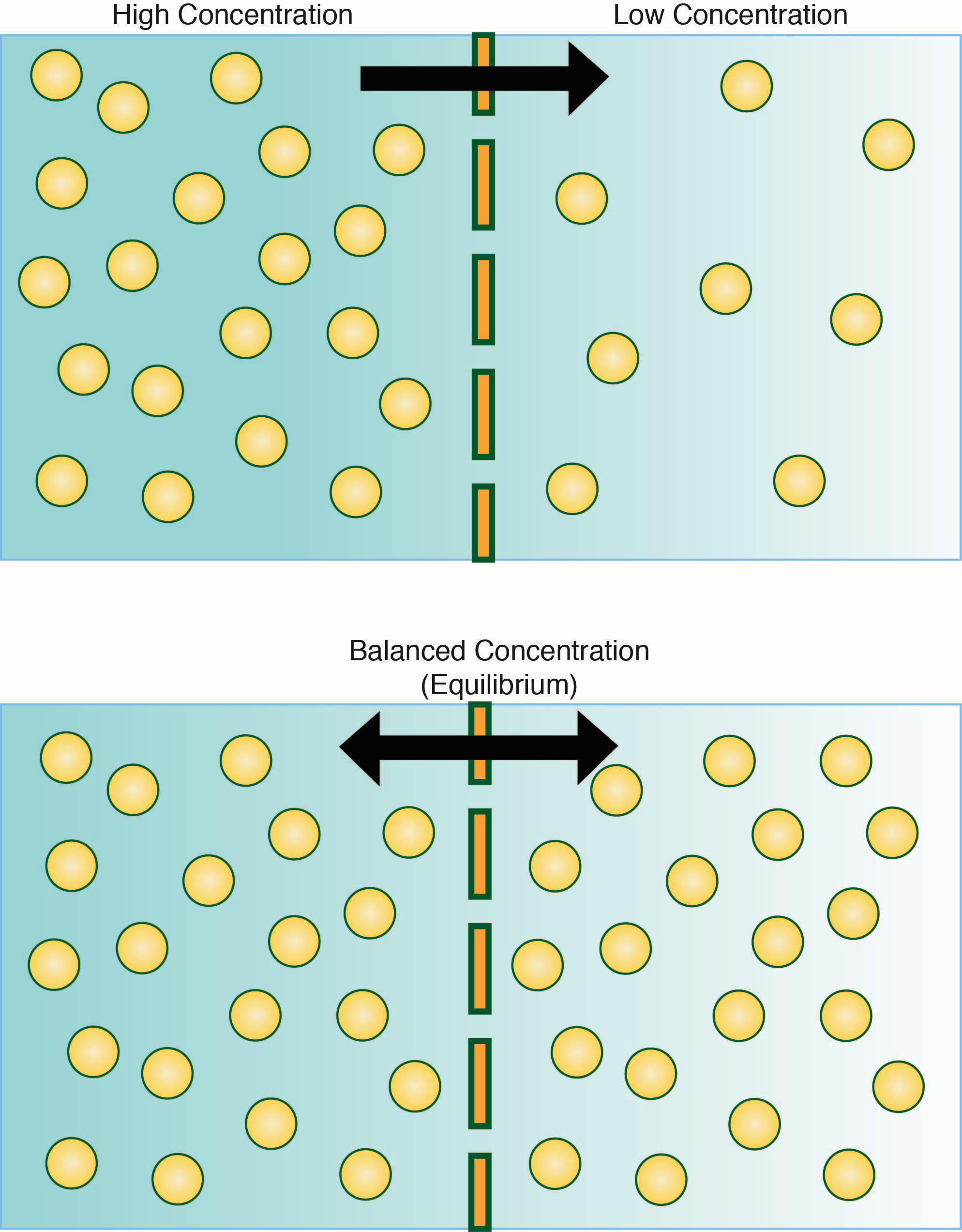
Membrane Transport Osmosis is the movement of free water molecules through a semipermeable membrane according to the water's concentration gradient across the membrane, which is inversely proportional to the solutes' concentration. in diffusion, the solutes move. in osmosis, the water moves. in both, the goal is the same: to balance out the solute concentration. Figure 6.5.1 6.5. 1: in osmosis, water always moves from an area of higher concentration (of water) to one of lower concentration (of water). in this system, the solute cannot pass through the selectively permeable membrane. a principle of diffusion is that the molecules move around and will spread evenly throughout the medium if they can. The rate at which water can move across cell membranes is increased by the presence of membrane proteins called aquaporins that form channels through which water molecules (but not solutes) can pass. osmosis refers to the passive movement of water across a semipermeable membrane (figure 7.6). Osmosis is a special case of diffusion. water, like other substances, moves from an area of high concentration of free water molecules to one of low free water molecule concentration. an obvious question is what makes water move at all? imagine a beaker with a semipermeable membrane separating the two sides or halves (figure 5.11).

Comments are closed.